INSTRUCTIONS
- Answer all the questions
- All working must be shown clearly where necessary
- Mathematical tables and silent non – programmable electronic calculators may be used
- Candidates may be penalized for not following the instructions given in this paper.
- Candidates must answer the questions in English.
- You are provided with
- Solid V
- 2.0 M hydrochloric acid, solution B
- 0.1M sodium hydroxide, solution C
You are required to determine the enthalpy change, ∆H for the reaction between solid V and one mole of hydrochloric acid
Procedure I
Using a burette, place 20 cm3 of 2.0M hydrochloric acid, solution B in a 100ml plastic beaker. Record the temperature of the solution after every half minute and record the values in table 1 below. At exactly 2 ½ minutes, add all of solid V to the acid. Stir the mixture gently with a thermometer and record the temperature of the mixture after every half minute in table 1. Retain the mixture for use in procedure II
Table 1
Time (Min)
0
1
1½
2
2½
3
3½
4
4½
5
Temperature
- On the grid provided, plot a graph of temperature (vertical axis) against time (3mks)
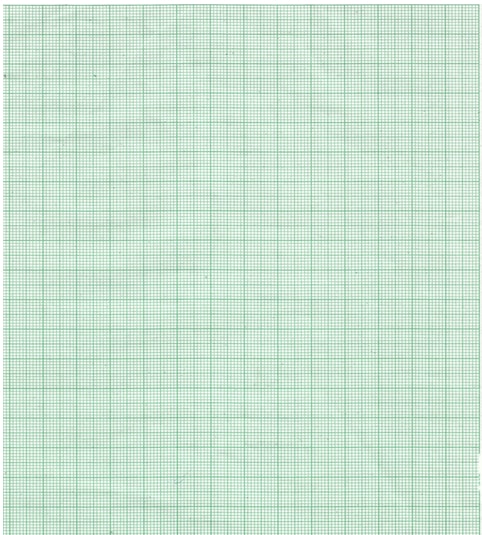
- From the graph, determine the change in temperature ∆T (1mk)
- Calculate the heat change for the reaction (Assume that the specific heat capacity of the mixture is 4.2Jk-1 1g-1 and the density of the mixture is 1g/cm3. (1mk)
Procedure II
Rinse the burette thoroughly and fill it with 0.1 M sodium hydroxide, solution C. Transfer all the contents of the 100ml plastic beaker used in procedure I into a 250ml volumetric flask. Add distilled water to make up to the mark. Label this solution V. Using a pipette and a pipette fillers, pipette 25cm3 of solution V into a conical flask . Add 2-3 drops of phenolphthalein indicator and titrate with solution C. Record your results in table 2 below. Repeat the titration two more times and complete table 2 below.
Calculate:I
II
III
Final burette reading
Initial burette reading
Volume of solution C used cm3
- The average volume of sodium hydroxide used (1mk)
- The number of moles of;
- Sodium hydroxide used (1mk)
- Hydrochloric acid in 25cm3 of solution V (1mk)
- Hydrochloric acid in 250cm3 solution V (1mk)
- Hydrochloric acid in 20cm3 of solution B, that reacted with solid V (1mk)
- Calculate the enthalpy of reaction between solid V and one mole of hydrochloric acid (2mks)
- You are provided with solid P. Carry out the tests below and record your observations and inferences in the spaces provided
- Transfer a spatula and full of solid P into a boiling tube and add 20cm3 of distilled water. Shake thoroughly then filter. Raise the residue and the distilled water and keep both the residue and the filtrate. Divide the filtrate into three portions.
- To one portion of the filtrate add ammonia solution drop wise until in excess
Observation Inference 1 mk ½ mk - To the second portion, add about four drops of 0.5M lead (II) nitrate solution
Observation Inference 1 mk 1 mk - To portion three of the filtrate, add 4 drops of Barium chloride solution followed by 5cm3 of nitric (v) acid.
Observation Inference 1 mk 1 mk
- To one portion of the filtrate add ammonia solution drop wise until in excess
- Scrap the residue from the filter paper in (a) above using a spatula and transfer it into a boiling tube. Add about 8cm3 of 1M nitric (v) acid.
Keep the resulting solution for use in (c) below
Observation Inference 1 mk 1 mk - Divide the solution obtained in (b) above into three portions
- To the first portion, add ammonia solution dropwise until in excess
Observation Inference 1 mk 1 mk - To the second portion, add 4 drops of 1M hydrochloric acid solution
Observation Inference 1 mk 1 mk - To the third portion, add 4 drops of Pottasium iodide solution
Observation Inference ½ mk ½ mk
- To the first portion, add ammonia solution dropwise until in excess
- Transfer a spatula and full of solid P into a boiling tube and add 20cm3 of distilled water. Shake thoroughly then filter. Raise the residue and the distilled water and keep both the residue and the filtrate. Divide the filtrate into three portions.
- You are provided with solid Q. Carry out the tests below and record you observations and inferences in the spaces provided.
- Scoop a portion of the solid using a clean spatula and introduce it to a non-luminous flame.
Observation Inference 1 mk 1 mk - To the remaining portion of solid Q, add 10cm3 of distilled water. Shake and divide the solution into three parts.
Observation Inference 1 mk 1 mk - To the first portion add about 1cm3 of acidified potassium manganate (vii) solution
Observation Inference 1 mk 1 mk - To the second portion, add about 1cm3 of acidified potassium dichromate (vi) solution
Observation Inference 1 mk 1 mk - To the third portion, determine the PH of the solution
Observation Inference ½ mk ½ mk
- Scoop a portion of the solid using a clean spatula and introduce it to a non-luminous flame.
Download CHEMISTRY PAPER 3 - 2019 KCSE Prediction Questions Set 2.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

