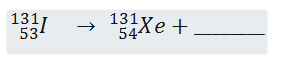Answer all questions
- The set up below can be used to prepare oxygen gas. Study it and answer the questions that follow.
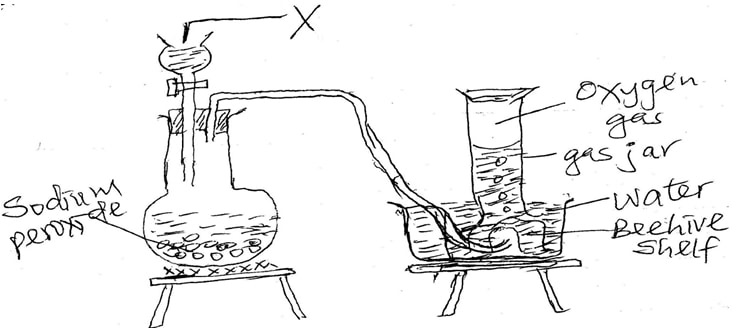
- Identify X ( 1mk)
- What property of oxygen makes it possible for it to be collected a shown in the above set up. ( 1 mk)
- State two uses of oxygen . ( 1mk)
- Write an equation to show the effect of heat on each of the following
- Silver nitrate ( 1mk)
- An hydrous iron (ii) sulphate ( 1mk)
- Sodium hydrogen carbonate .( 1mk)
-
- What name is given to the process by which alcohol is formed from a carbohydrate.?. (1mk)
- Explain why the solubility of ethane in water is lower than that of ethanol. ( 2mks)
-
- Complete the nuclear equation below (1mk)
- The half – life of is 8 days
Determine the half life of if 50 grammes decayed for 40 days. ( 1mk) - Give one agricultural use of radio isotope. ( 1mK)
- Complete the nuclear equation below (1mk)
- Charcoal is a fuel that is commonly used for cooking. When it burns it forms two oxides
- Name the two oxides . (2mks)
- State one use of any of the two oxides . (1mk)
- An element X has a relative atomic mass of 88. When a current of 0.5 amperes was passed through a fused chloride of X for 32 minutes, 10 seconds; 0.44g of X was deposited.
- Determine the charge of element X (1 Faraday = 96,500c) . ( 2mks)
- Write the formula of hydroxide of X. ( 1mk)
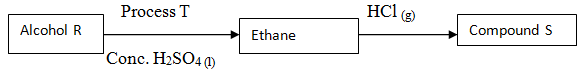
- Study the following flow chart and answer the questions that follow:
- Write the formula of
- Alcohol R (1mK
- Compound S (1mk)
- Name process T ( 1mK)
- Write the formula of
- 60cm3 of oxygen gas diffuses through a porous plug in 50 seconds. How long will it take 80cm3of sulphur ( iv) oxide to diffuse through the same plug under the same conditions. (S = 32 O = 16 ) ( 3mks)
- Study the information in the table below and answer the questions that follow.
Salt
Solubility
g/100g water
At 50o C
At 80oc
G
43
58
Y
82
138
A mixture containing 40g salt G and 120g salt Y in 100g of water at 80o was cooled to 50o- Which salt crystallized out?. Give a reason (2mks)
- Calculate the mass of the salt that crystallized out. (1mk)
-
- State two conditions necessary for rusting to occur.(1mk)
- State the two reasons why tin coating is used in food cans. (2mks)
- A form one student was supplied with a colourless liquid which was suspected to be water.
- Describe one chemical test that could be carried out to show that the liquid is water.(2mks)
- How could it have been shown that one liquid was pure water (1mk)
- During extraction of copper, the ore is first concentrated and roasted to produce copper (I) Sulphide.
- Name the ore from which copper is commonly extracted. (1mk)
- Write an equation for the reaction in which copper (I) sulphide is produced by roasting the ore in air .(1mk)
- Give one effect that the process in (ii) above could have on the environment. ( 1mk)
- Give one use of copper metals (1mk)
-
- Name two cations that are present in hard water. (1mk)
- Explain how the ion exchange resin softened had water.(2mks)
- Below is a representation of an electrochemical cell.
Pb (s)/ Pb 2+(aq) // Ag+(aq) / Ag(s)- What does / / represent ? ( 1mk)
- Given the following: EƟ (V)
Pb2+(aq) + 2e → Pb(s) - 0.13
Ag+(aq) + e → Ag (s) + 0.80
Calculate the e.m.f of the electrochemical cell. (2mks)
- Distingush between ionization energy and electron affinity of an element (1mk)
- Calculate the percentage by mass of copper in copper (ii) carbonate salt.
( Cu = 64, C= 12, 0 = 16 ) ( 3mks) - What name is given to elements which appear in group (II) of the periodic table? ( 1mk)
- Explain why the following substances conduct an electric current
- Magnesium metal ( 1mk)
- Molten magnesium chloride ( 1 mk)
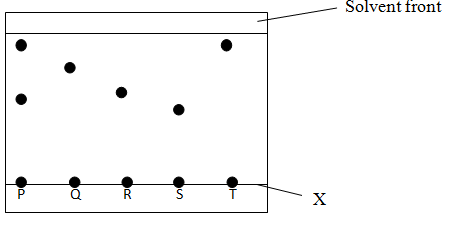
- The chromatography below was obtained from a contaminated food sample P. Contaminants Q, R, S and T are suspected to be in P. Use it to answer the following questions.
- Name line labelled X. ( 1mk)
- Identify the contaminants in mixture P.( 1mk)
- Which is the most soluble contaminant in P. ( 1mk)
-
- Diamond and graphite are allotropes of carbon. What is meant by an allotrope? (1 mk)
- Explain why graphite can be used as a lubricant while diamond cannot. (2mks)
- Where 15cm3 of a gaseous hydrocarbon,p, was burnt in 100 cm3 of oxygen, the resulting gaseous mixture occupied 70cm3 at room temperature and pressure. When the gaseous mixture was passed through potassium hydroxide solution, its volume decreased to 25cm3 .
- What volume of oxygen was used during the reaction?. (1mk)
- Determine the molecular formular of the hydrocarbon. (2mks)
- In terms of structure and bonding, explain the following observations:
- The melting point of aluminium is higher than that of sodium. (1½ mks)
- Melting point of chlorine is lower than that of sulphur. (1½ mks)
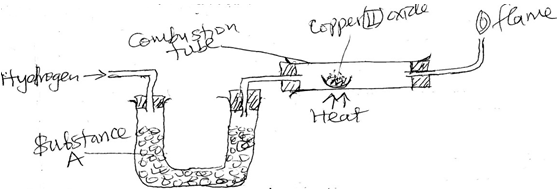
- The set up below was used to investigate the reaction between dry hydrogen gas and copper (II) oxide.
- Name substance A. ( 1mk)
- State the observation made in the combustion tube. (1mk)
- Explain the observations made in (ii) above. (1 mk)
- Hydrogen chloride gas can be prepared by reacting sodium chloride with an acid.
- Write an equation for the reaction between sodium chloride and the acid. ( 1mk)
- Give two chemical properties of hydrogen chloride gas (1mk)
- State two uses of hydrogen chloride gas. (1mk)
- State and explain what would happen if a dry red litmus paper was dropped in a gas jar of dry chlorine. (2mks)
- By using aqueous sodium chloride describe how a student can distinguish calcium ions from lead ions. (2mks)
- Given the following substances: wood ash lemon juice and sodium chloride
- Name one commercial indicator that can be used to show whether wood, lemon juice and sodium chloride are acidic, basic or neutral. (1mk)
- Classify the substances in 27(a) above as acids, bases or neutral.(2mks)
28.A solution was made by dissolving 8.2g of calcium nitrate to give 2 litres of solution. Determine the concentration of nitrate ions in moles per litre. (3mks)
Download CHEMISTRY PAPER 1 - 2017 MURANG'A MOCK EXAMINATION.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students