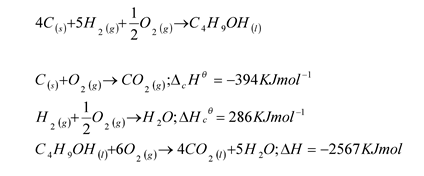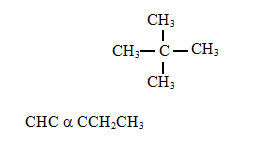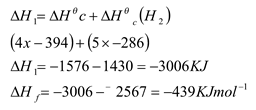INSTRUCTIONS TO CANDIDATES
- Answer all the questions in the spaces provided.
- All working must be clearly shown where necessary.
- KNEC mathematical tables and silent non-programmable electronic calculators may be used.
- Candidates should check the question paper to ascertain that all the pages are printed as indicated and that no questions are missing.
- Candidates should answer all the questions in English
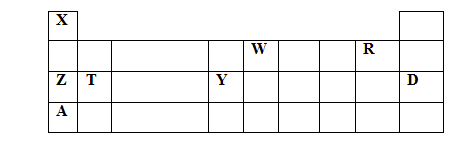
- The grid below is a section of the periodic table , the letters are not the actual chemical symbols. Use it to answer the questions that follow.
- To which chemical family do the elements Z and A belong ? (1 mk )
- Write a balanced chemical equation for the reaction between T and Steam ( 1mark)
- compare
- the atomic radius of W with that of R. Explain. (2 mks)
- the reactivity of Z and A. Explain. (2 mks)
-
- element X can also be placed above element R. Explain. (1mk)
- Name one use of element D. (1 mk )
- Which element Forms an oxide that reacts with both aqueous solutions of sodium hydroxide and hydrochloric acid. Explain. (2marks)
- Write a balanced equation to show the effect of heat on the
- Nitrate of A (1mark)
- Carbonate of T (1 Mk)
- write an ionic equation for the reaction between the aqueous solutions of the chloride of T and hydroxide of Z. (1 mk)
-
- Define
- Endothermic reaction (1mk )
- Molar heat of vaporization (1mk)
-
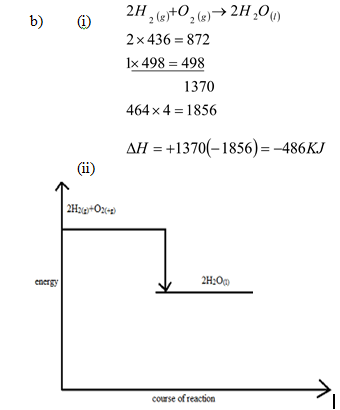
- Using bond energies given below, calculate the enthalpy change for the reaction of oxygen gas and hydrogen gas to form water (2mks)
Bond
Bond energies KJmol-1
H – H
O = O
O – H
436
498
464
- Draw an energy level diagram for the reaction in (i) above. (2mks)
- Using bond energies given below, calculate the enthalpy change for the reaction of oxygen gas and hydrogen gas to form water (2mks)
- Given the equation for the formation of Butanol and the enthalpy changes below;
- Draw an energe cycle diagram to represt the information 2mks
- calculate the standard enthalpy change for the formation of butanol 1mk
- Explain how the value of molar heat of neutralization obtained when 100 cm 3of 1 M hydrochloric acid reacts with 100 cm3 of 1 M sodium hydroxide compares with the one that would be obtained if the experiment was repeated using 100 cm 3of 1 M ethanoic acid instead hydrochloric acid (2mks)
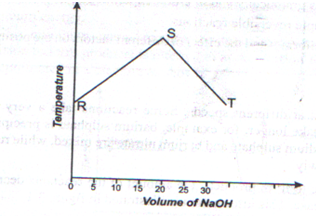
- The graph below shows the change in temperature when 0.5 M NaOH solution was added to 20 cm3 of HCl.
- What does point S represent? (1mk)
- Why does the temperature rise from R to S? ( 1mk)
- Determine the molarity of the HCl acid (2 mks)
- Define
- A group of form four students carried out an experiment to determine the solubility of potassium chlorate. The table below shows the results obtained.
Total volume of water added(cm3)
10.0
20.0
30.0
40.0
50.0
Mass of KClO3(g)
5.0
5.0
5.0
5.0
5.0
Temperature at which crystals appear(0C)
80.0
65.0
55.0
45.0
30.0
Solubility of KClO3(g/100gH2O)
- Complete the table to show the solubility of KClO3 at different temperatures. (3mks)
- Plot a graph of mass of KClO3 per 100g water against temperature at which crystals form.(3mks)
- From the graph, determine ;
- The solubility of KClO3 at 40o (1mk)
- The temperature at which the solubility of KClO3 is 35g/100g water. (1mk)
- Explain the shape of the graph. (1mk)
- State one application of solubility and solubility curves. (1mk)
- In an experiment, soap solution was added to three separate samples I,II,III of water. The table below shows volumes of soap solution required to form lather with 1000cm3 of each sample of water before and after boiling.
SAMPLE
I
II
III
Volume of soap before water is boiled (cm3)
25.0
5.0
10.0
Volume of soap after water is boiled(cm3)
25.0
5.0
5.0
- Which water sample was likely to be soft? Explain. (1mk)
- Explain the change in volume of soap solution used in sample III (2mk)
- name the two cations that make water hard. 1 mk )
- which water sample likely contained sulphate ions 1 mk )
- name one advantage of using hard water. ( 1 mk)
-
- A student wrongly categorised air as a compound and not as a mixture. Give two reasons as to why the student was wrong. ( 2 mks ) (2mks)
- The table below shows the results obtained when four solvents were used to separate a dye. Study the results and use them to answer the questions thatfollow.
Solvent
Number of Solute components
A
5
B
1
C
D
0
2
- Identify the most suitable solvent for this separation. Give a reason for your answer. (2mks)
- What does the result of the solvent C tell us about the dye? (1mk)
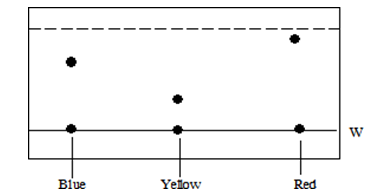
- The chromatogram below was obtained from a plant extract. Use it to answer the questions that follow.
- Name line W(1 mk)
- What does the dotted line represent? 1mk (1mk)
- State with a reason the least soluble dye in the moving solvent.. ( 2 mks) (1mk)
- Below is a list of major component of crude oil and their boiling points.
Component Boiling point (0C)
Bitumen Above400
Lubricatingoil 350 -400
Petrol 40 -175
Gases Below40- Name is the name of the process by which the constituents of crude oil can be separated? (1mk)
- Give one use of the gases component.(1 mk)
- Give the order by which the components are obtained from the mixture, starting with the first. (1mk)
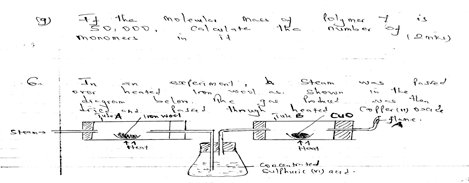
- In the experiment, steam was passed over heated iron wool as shown in the diagram below. The gas produced was then dried and passed through heated copper (ii) oxide
- Write an equation for the reaction between steam and iron 1mk
- What observation would be made in tube B at the end of reaction? Explain 2mks
- What precaution should be taken into consideration before lighting the gas at A 1mk
- What type of reaction takes place in the tube B 1mk
- Give TWO uses that are for both carbon(ii) oxide and hydrogen gases 2mks
- Give the name of the process described below 3 mk
Substance
condition
Name of the process
Hydrated iron (ii) sulphate
Exposed to air ,changes from crystalline to powder form
Concentrated sulphuric(vi) acid
Exposd to air, volume of the acid increases
Zinc nitrate
Exposed to air changes in solution
- Name another substance that can undergo the same process as zinc nitrate above 1mk
- Write the formula of copper (ii) sulphate crystals 1mk
- Give the name of the process described below 3 mk
-
- Chlorine gas can be prepared in the laboratory by reacting Manganese (IV) oxide with concentrated hydrochloric acid.
- write a chemical equation for the reaction above ` (1mark)
- what process does concentrated hydrochloric acid undergo to produce chlorine gas? Explain. (1 mks)
- Chlorine is a strong oxidizing agent. Write down an equation for the reaction of Chlorine with
- Hydrogen sulphide gas. (1mark)
- with iron (ii) chloride (1mark)
- Hydrogen Chloride gas is a colourless gas which dissolves readily in water forming hydrochloric acid.
- At room temperature and pressure, 1.00dm3 of water dissolves 432 dm3 of hydrogen chloride gas. How many moles of hydrogen chloride dissolves in 1dm3 water ( 1 mole at r.t.p. occupies 24.0dm3) (1marks)
- The hydrochloric acid formed has a volume of 1.40 dm3; calculate the concentration of the acid in mol/dm3. (1mark)
- In the solution, the molecules ionize as below
HCl(ag) →H+(ag) + Cl-(ag)
Describe a simple test to confirm presence of Cl- ions in the solution. (1marks)
- Chlorine gas can be prepared in the laboratory by reacting Manganese (IV) oxide with concentrated hydrochloric acid.
- below are molecules of hydrocarbons.
- What are hydrocarbons? ( 1 mk)
Name the following compounds. - What observation would be made if acidified potassium manganate (vii) is added to the solution in b above. Explain. (2 Mark)
- What are hydrocarbons? ( 1 mk)

MARKING SCHEME
-
- Alkali metals
- T(s) + H2O (g)→TO(s) + H2(g) OR Mg(s) + H2O(g) → MgO(s) + H2(g)
-
- W is greater than that of R, W has fewer protons hence has a smaller force of attraction to the electrons.
- A is more reactive than Z, A is more electropositive. A has a large atomic radius than Z hence looses electrons more easily.
-
- X gains one electron to become stable ,just like group vii elements
- in filling electric bulbs/ to provide an inert surrounding in bulbs
- Y, its oxide is amphoteric
-
- 2ANO3(s) →2ANO2(s)+O2(g) / 2KNO3(s) →2KNO2(s) + O2(g)
- TCO3(s) TO(s) + CO2(g) /MgCO3(s) MgO(s) + CO2(g)
- T2+ (aq) + 2OH- T(OH)2(s) / Mg2+ (aq) + 2OH(aq)- Mg(OH)2(s)
-
-
- Endothermic reaction : reaction where energy is absorbed (taken in )from surrounding.
- The amount of heat required to change one mole of a liquid into vapour at constant temperature (at it’s B.P).
-
-
- The value when ethanoic acid is used would be lower because it is a weak acid and therefore some energy wouid be use in ionizing it-
-
- point at which complete neutralization takes plac
- there is increase in the number of molesof sodium hydroxideneutralised as volume as volume of sodium hydroxide increases
- Number of moles of of NaOH =20×0.5/1000, =0.01 moles
mole ratio NaOH : HCl is 1:1
hence No of moles in 20 Cm Hcl= 0.01 moles
No of moles in 1000Cm3 HCl=0.01×1000/20=0.5
Molarity of HCl=0.5 M
-
-
- II – little and constant amount of soap was used before and after boiling. (1 mk)
- Contain temporary hardness which can be removed by boiling. (1 mk)
- Mg2+ and Ca2+ ions or magnesium and calcium ions. (1 mk)
- Sample 1. (1 mk)
-
- Bone formation
- used brewing
- leather industry Any (1 mk)
-
- compound cannot.
- The components of air are not in definite whole number ratio in a compound, the elements are combined in definite whole number ratios,
- A compound is a pure substance whilst air is a mixture of several substances. (any (2 x 1) =2mks
- The component of air can be separated by physical means while that of compound can not.
-
- A : It separates the dye to the greatest number of components ie separates the dye the most.
- The dye is insoluble in the solvent C
-
- Baseline /Origin
- The solventfront
- Yellow - It moves the shortest distance from theorigin.
-
- Fractionaldistillation
- The gases are used as afuel
- Gases, Petrol, Lubricating oil, Bitumen
- compound cannot.
-
Total volume of water added(cm3)
10.0
20.0
30.0
40.0
50.0
Mass of KClO3(g)
5.0
5.0
5.0
5.0
5.0
Temperature at which crystals appear(0C)
80.0
65.0
55.0
45.0
30.0
Solubility of KClO3(g/100gH O)
50.0√½
25.0√½
16.7√½
12.5√½
10.0√½
NB: √½mk for expressing solubility values to the same number of decimal points
2 mks at least fair correct- Graph marking points: scale (graph to cover ˃= ½ grid)√½
Labeling of axes√½
Plotting of points √1
Curve (smooth) √1 -
- 11g/100gH2O √1 ½ mk for showing on graph and ½ correct figure 1mk
- 720C (+/- 0.5)√1 ½ mk for showing on graph and ½ mk for correct value 1mk
- Solubility of KClO3 increases with increase in temperature/more KClO3dissolves as temperature rises√1
- Extraction of soda ash(Sodium carbonate) from Trona√1
- Extraction of common salt(sodium chloride). Etc
- Graph marking points: scale (graph to cover ˃= ½ grid)√½
-
- 3Fe(s) +4H2O(g) → Fe3O4(s) + H2(g)
- droplets of colourless droplets on upper colour path of the hydrogen is oxidized by CuO - Black CuO turns red-brown/brown. Hydrogen reduces (01) CuO to copper metal
- Excess hydrogen gas should be passed before lighting it to avoid explosion
- Reduction
- They are both used as fuel 01
- they are both used as reducing agents of some metallic oxide 01 -
- efflorescence (1 mk)
hygroscopy (1 mk)
deliquescence (1 mk) - Sodium hydroxide, iron (iii) chloride . (1 mk)
- CuSO4 SH2O
- efflorescence (1 mk)
- MnO2(s) + 4 HCl (l) →MnCl2(aq) + Cl2 (g) + 2H2O(l)
Oxidation, HCl has lost hydrogen to form chlorine gas OR Chlorine has its oxidation number increase from -1 to O -
- H2S (g) + Cl2(ag)→ 2 HCl(g) + S(s)
- 2 Fe Cl2(ag) + Cl2(g) → 2 FeCl3(ag)
-
- 24dm3 = 1 mol
432dm3 = 1 x 432 = 18 moles - 1.40dm3 = 18.0
1 dm = ?
1 dm2 = 18 x 1 = 12.86 M
1.4 - Add a few drops of acidified AgN03or acidified
Pb(NO3)2 in a test tube containing Cl- ions
A white precipitate is formed.
In Pb(NO3)2 the precipitate dissolve on warming
- 24dm3 = 1 mol
- 3Fe(s) +4H2O(g) → Fe3O4(s) + H2(g)
-
- molecules/ compounds made up of carbon and hydrogen only.
-
- 2,2-dimethyl propane
- Pent-2-yne
- compound 1 does not decolourise purple KMnO4 while compound 2 does, compound 2 is unsaturated
Download CHEMISTRY PAPER 2 - 2019 KCSE CEKENA MOCK EXAMINATION (QUESTIONS AND ANSWERS).
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students