Instructions to candidates
- Answer all questions in this question paper.
- Answers to all questions must clearly be written
- All working MUST be clearly shown where necessary.
- KNEC mathematical tables and non-programmable silent electronic calculators may be
- In an experiment of diluting concentrated sulphuric (vi) acid, 2 cm3 of acid were carefully poured into a plastic cup containing exactly 40cm3 of distilled water with a room temperature of 200c. the mixture was stirred with a thermometer; the highest temperature noted was 350c. (density of acid = 1.84g/cm3 while that of solution is assumed to be 1g/cm3. The acid is 98% pure, S.H.C. =4.2J/g/k (H = I S = 32 O = 16)
-
- Determine the number of moles of the acid that dissolved (2 mks)
- Determine the enthalpy change for the reaction. (2mks)
- Determine the enthalpy change when one more of the acid is dissolved in water. (2 mks)
- use the information below to answer the questions that follow;
Equation Enthalpy of formation
C(s) + O2(g) →CO2(g) ΔH1 = -394KJMol-
C(S) + o2(g)→CO(g) ΔH2 =-170KJMol- - Define the term enthalpy of formation of a compound (1mk)
- Calculate the molar enthalpy of combustion : ΔH3 of carbon (ii) oxide (3mks)
-
-
- Name the following compounds (3 mks)
- CH3CH2CH2OH
- CH3 CH2 CH2 COOH
- CH3CH2CH2COOCH2CH3
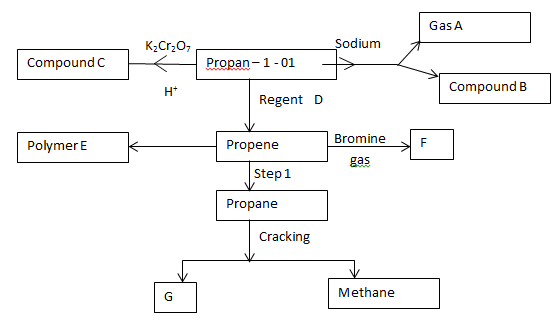
- Study the scheme below and answer the questions that follow
- Identify product
A (1 mk)
F (1 mk) - Name the compound C (1 mk)
- State the conditions for step 1 (1 mk)
- Name the process leading to formation of compound C (1 mk)
- Write an equation for the reaction leading to the formation of methane. (1 mk)
- Identify reagent D. (1 mk)
- Draw the structure of F. (1 mk)
- Identify product
- Name the following compounds (3 mks)
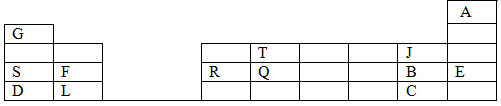
- The grid below is a section of the periodic table (letters used are not actual symbol) use it to answer questions that follow.
- Select the most electro-negative element. (1 mk)
- The boiling point of the oxide of Q is much higher than that of the oxide of T. Explain the difference (2 mks)
- Identify with a reason the chemical family to which F and L belong. (2 mks)
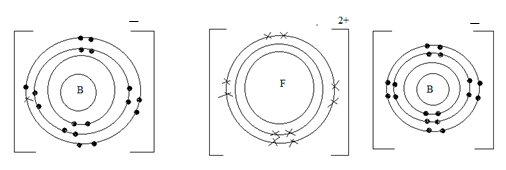
- Use dot (.) and cross (x) diagram to show bonding in the compound formed when F reacts with B. (1 mk)
- State and explain the nature of chloride of R when it is dissolved in water to form an aqueous solution. (2 mks)
- Compare the atomic radius of elements D and L. (2 mks)
- The elements S and D belong to group I, which element is more reactive, explain. (2 mks)
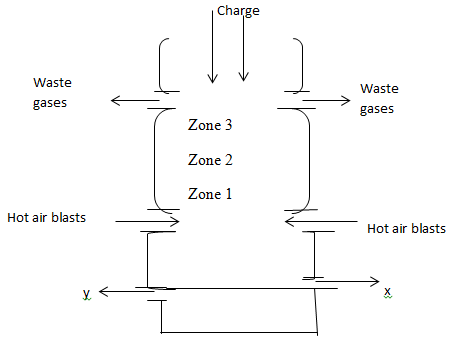
- The diagram below shows a blast furnace in the extraction of iron from haematite.
- Name two other ores that can be used to extract iron. (2mks)
- Name the components of the charge (raw materials). (1 mks)
- Identify two components of the waste gases. (1 mks)
- Give the identity of X and Y. (2 mks)
- Identify two reducing agents in the blast furnace. (1 mks)
- Write the chemical equation for the reduction of haematite to iron metal using the main reducing agent. (1 mk)
- Which zone is the hottest? Explain. (1 mks)
- The flow chart below represents some industrial processes leading to the formation of two nitrogenous fertilizers.
- Name the catalyst used in (2 mks)
- Process 2
- Ostwald’s process
- Name each of compounds X and Y (1 mk)
- Other than the catalyst named in (b) above, state two optimum conditions for process labeled 2. (1 mk)
- Briefly describe process 1 that leads to production of nitrogen from air. (3 mks)
- Other than ammonium nitrate being used as a fertilizer name one other use (1 mks)
- Ammonium nitrate and ammonium sulphate are used as fertilizers, one would you recommend to a farmer and why? show your working (N=14,O=16,S=32,H=1) (3MKS)
- Write an equation for the formation of Sulphur( iv) oxide in contact process. (1mk)
- Sulphur ( iv) oxide is an acid anhydride of sulphuric vi acid, but in contact process Sulphur (iv) oxide is first dissolved in sulphuric (vi) acid. Explain why this is so. (2mks)
- Name the catalyst used in (2 mks)
-
- The table below gives standard electrode potentials for the metals represented by the letters R, S, T and U. study and answer the questions that follow.
METALS Standard Electrode Potential (Volts)
R - 0.34
S - 0.85
T + 0.34
U - 0.76- Identify the metal which is the strongest reducing agent (1 mk)
- Which metal can be displaced from a solution of its salts by all the other metals in the table? Give a reason (2 mks)
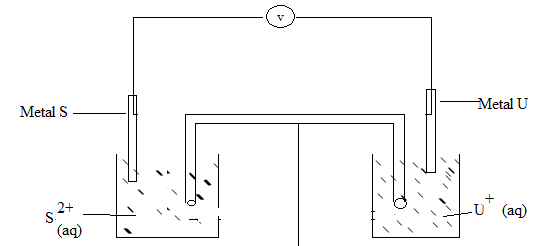
- Metal S and U were connected to form a cell as shown in the diagram below.
- Write the equation for the cell above (1 mk)
- Calculate the e.m.f, for the cell above (1 mk)
- On the diagram, indicate with an arrow the direction in which electrons would flow on the diagram above (1 mk)
- State one function of the salt bridge. (1 mk)
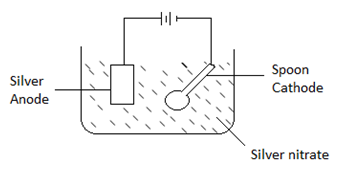
- In an experiment to electroplate a copper spoon with silver, a current of 0.5 A was passed for 18 minutes.
- Draw a well labeled diagram showing how the copper spoon was electroplated. (2 mks)
- Other than electroplating state one use of electrolysis (1 mk)
- The table below gives standard electrode potentials for the metals represented by the letters R, S, T and U. study and answer the questions that follow.
- A group of form four students of Cockelbet Secondary School carried out an experiment to determine the solubility of potassium chlorate. The table below shows the results obtained.
Total volume of water added(cm3) 10.0 20.0 30.0 40.0 50.0
Mass of KClO3(g) 5.0 5.0 5.0 5.0 5.0
Temperature at which crystals appear(0C) 80.0 65.0 55.0 45.0 30.0
Solubility of KClO3(g/100gH2O)- Complete the table to show the solubility of KClO3 at different temperatures. (3mks)
- Plot a graph of mass of KClO3 per 100g water against temperature at which crystals form. (3mks)
- From the graph, determine ;
- The solubility of KClO3 at 40 0C. (1mk)
- The temperature at which the solubility of KClO3 is 35g/100g water. (1mk)
- Explain the shape of the graph. (1mk)
- State one application of solubility and solubility curves. (1mk)
- In an experiment soap solution was added to three separate samples of water. The table below shows the volumes of soap solution required to form lather with 100cm3 of each sample of water before and after heating/boiling
SAMPLE
A
B
C
Volume of soap before water is boiled in (cm3)
30
4
12
Volume of soap after water is boiled in (cm3)
30
4
4
- Which water sample is likely to be soft. Explain (2MKS)
- Explain the change in the volume of soap solution used in sample C (1MK)

MARKING SCHEME
-
-
- 2cm3 x 1.84g/cm3=3.68g ½ mk
98/100 x 3.68=3.6064 ½ mk
3.604/98 = 0.0368 moles ½ mk - McΔT ( ½ mk)
42g x 4.2 x 15k ( ½ mk) =2646J
=2.646Kj 1mk - 0.0368 moles – 2.646 J ( ½ mk)
1 mole
2.646 = -71.739 kJ mole ( ½ mk)
penalize ½ for missing units/ sign
0.0368
- 2cm3 x 1.84g/cm3=3.68g ½ mk
- Is heat evolved when one mole of a compound is formed from its constituents elements
- ΔH3=ΔH1-ΔH2
= -394-(-170)= -224kJMol-1
-
-
-
- Butanol
- propanoic acid
- Ethyl butanoate
-
- hydrogen gas
F-1,2 dibromo propane. - C-propanoic acid
- Nickel catalyst ½ mk
150-250ᴼC ½ MK - Oxidation
- C3H8(g)→ CH4(g)+ C2H4(g)
- Conc H2SO4
-
- hydrogen gas
-
-
- J
- Q Has a giant atomic structure ( ½ mk) with covalent bonds between the atoms while T has weak vander waals forces between its molecules
- Alkaline earth metals. They are found in the earth’s crust and form alkaline solutions when dissolve in water.
-
- Acidic ( ½ mk), it gets hydrolyzed to by water producing HCL. ( ½ mk)
- D is larger than L ( ½ mk) since it has fewer protons hence nuclear charge/ attraction towards electron is weaker. ( ½ mk)
- D, ( ½ mk) it has a larger atomic radius hence loses outer most electron most readily. ( ½ mk)
-
- Magnetite , Siderite
- Ore, limestone, coke
- CO2CO
- X-Slag
y-molten iron - C, CO
- Fe2O3(S) +3CO(g)→ 2Fe(l)+3CO2(g)
- Zone 1.reaction that occurs is exothermic.
-
-
- Iron
- Platinum
- X-Nitric (v) acid
Y-Ammonia - Temp 400ᴼC-500ᴼC ½ mk
Pressure 200-500 atmospheres ½ mk - - Pass air through purifiers (to remove dust)
- then pass air through sodium hydroxide to remove co2
- cool air to -250c to remove water vapour
- cool it to -2000c to liquefy it, fractional
- distillation is done on liquid air and nitrogen gas is collected at -1960c
( ½ mk each = 3mks) - To manufacture fertilizers e.g. C.A.N.
- the reaction between SO3 and water is highly exothermic hence the heat produced vapourises the acid
- ammonium nitrate,
28/80×100=35%
Ammonium sulphate
28/132×100=21.2%
Ammonium nitrate would be recommended - 2 SO2(g) +O2(g) → 2SO3(g)
- The reaction is exothermic
-
-
-
- S
- T-is the most electronegative
-
- S(S) +2U+(aq)→S2+(aq)+2U(S)
- E red –E or -0.76+0.85=+0.09v
-
-
- Complete the circuit
- To balance the charges in the electrolytes
-
-
- Purification of less reactive metals e.g sodium
- Extraction of reactive metals e.g. Na
-
-
-
-
50
25
16.67
12.5
10
- P=All plots correct 1 mk
4 plots corret 1/2mk
Scale= 1MK
Curve =1 - solubity increases with increase in temperature
- Extraction of sodium chloride separation of ammonium chloride and sodium hydrogen carbonate in solvay process
- 1 B; because the volume of soap used is 4cm3before and after is same
- temporary hardness was removed by boiling
-
Download CHEMISTRY PAPER 2 - 2019 KCSE KASSU JOINT MOCK EXAMS (QUESTIONS AND ANSWERS).
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students