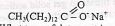Instructions to Candidates:
- Write your name and index number in the spaces provided above.
- Sign and write the date of examination in the spaces provided above.
- Answer ALL the questions in the spaces provided in the question paper.
- Mathematical tables and silent electronic calculators may be used.
- All working MUST be clearly shown where necesiary.
- Candidates should check the question paper to ascertain that all pages are printed as indicated and that no questions are missing.
For Examiner's Use Only:
| QUESTIONS | Max. score | Candidates score |
| 1-27 | 80 |

QUESTIONS
- Manganese (IV)oxide is one of the compound used in the preparation of oxygen gas.
- Write down the chemical formula of Manganese(IV) oxide. (1mk)
- Give one use of Manganese (IV) oxide in the preparation of oxygen gas (1mk)
-
- Name two types of flames produced by a Bunsen burner (2marks)
- Describe how luminosity of a flame can be increased (1mk)
-
- Define the following terms;
- Lattice energy(1mk)
- Hydration energy(1mk)
- Given the following , find the lattice energy of potassium bromide
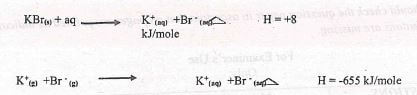 (1mk)
(1mk)
- Define the following terms;
-
- State Boyle's law (1mk)
- A gas occupies 650cm3 at a pressure of 760mmHg. If the pressure is raised to 1220mmHg,what volume would the gas occupy? (2mks)
-
- The following numbers represents pl values of some substances, 2, 4, 5, 7, 9 and 13.
Select the appropriate pil for each of the following- Sour milk .(1mk)
- Wood ash solution (1mk)
- A gas is prepared by adding concentrated Sulphuric (VI) acid to Sodium chloride crystals .Q is denser than air and dissolves in water to form a solution of pl less than 3. Name gas (1mk)
- Which property of concentrated Sulphuric (VI) acid is applied in the preparation for gas Q. (1mk)
- The following numbers represents pl values of some substances, 2, 4, 5, 7, 9 and 13.
- 30.0cm3 of sodium hydroxide solution was diluted to 810cm? 27.0cm of the diluted solution required 25.Och of 0.054M sulphuric (VT) acid for complete neutralization. Determine the mass of sodium hydroxide in 30.0cm of solution. (Na-23,0=16, H=1.0)(3marks)
- A student used the set up shown in the diagram below in order to study the reactions of some metals with steam. The experiment was carried out for ten minutes.
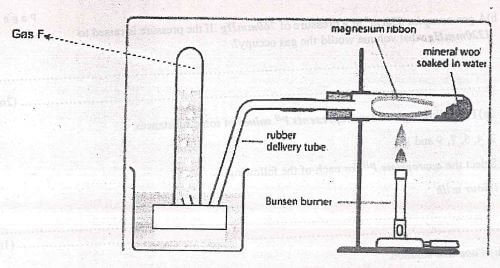
- What observation would be made if gas F is ignited ? (1mk)
- When the experiment was repeated using iron powder instead of magnesium ribbon , very little gas F was obtained.
- Give a reason for this observation. ..........(1mk)
- What change in conditions of the experiment should the student have made in order to increase the volume of gas F produced. ... (1mk)
- Element Y is made up of three isotopes; 20Y,21Y and 22Y with percentage abundance of (x +82.1)%, 0.26% and x% respectively The relative atomic mass of element Y is 20.179.
Calculate the percentage abundance of isotope 22Y (3marks) - State and explain the differences in melting point of;
- Sodium and Aluminium.(1 marks)
- Oxygen and Sulphur ............. (11/2marks)
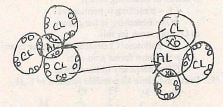
- Using dots() and cross(x) diagram; show the compound formed when Aluminum chloride is in vapour form. (3marks)
- Copper() sulphate solution forms a pale blue precipitate when reacted with aqueous ammonia. The precipitate dissolves in excess ammonia to form a deep blue solution.
- Identify the pale blue precipitate ...(1mk)
- Write an ionic equation for the formation of the deep blue solution ....(1mk)
- A mixture contains Ammonium chloride, Copper (II) oxide and Sodium chloride. Describe how each of the substances can be obtained from the mixture.................. (3marks)
- The following apparatus shown below was used to investigate the effect of Carbon(II)oxide on Copper(II)oxide
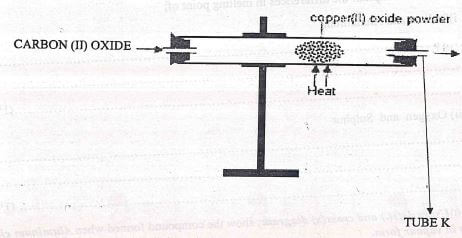
- State the observation that was made in the combustion tube at the end of the experiment...............(1 mk)
- Write an equation for the reaction that took place in the combustion tube. c)Why is it necessary to burn the gas coming out of the tube K? ... (1mk)
- The graph below shows the relationship between pressure and the temperature of a gas in a fixed volume of a container.
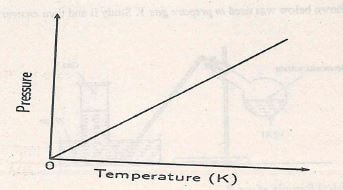
- State the relationship between temperature and pressure that can be deduced from the graph. (1mk)
- Using kinetic theory, explain the relationship shown on the graph.... (2mks)
- A pellet of Sodium Hydroxide left exposed to air underwent the following changes:
- Changed into a colourless liquid, then
- Formed colourless transparent crystals and finally
- The crystals formed a white powder.
- Use one word to describe each of the changes in(i) and (iii)
- ....(1mk)
- ....(1mk)
- Use one word to describe each of the changes in(i) and (iii)
-
- Name a reagent that can use to distinguish between Al3+(aq) and Zn2+(aq) ions (1mk)
- State what is observed if each ion is treated separately with the reagent. (1mk)
- The set up shown below was used to prepare gas Y. Study it and then answer the questions that follow
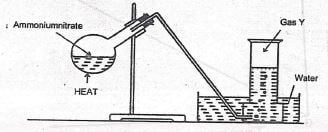
- Give the chemical formula of gas Y (1 mk)
- Give the confirmatory test for gas Y (1 mk)
- State one use of gas Y ...(1mk)
-
- Write an equation for the reaction between hot concentrated Sodium hydroxide and Chlorine gas. ...(1mk)
- Chlorine gas bleaches by oxidation while Sulphur(IV)oxide by reduction. Give any other difference between the two gasses when it comes to bleaching action. .................(1mk)
- Massive emission of Chlorine gas into the environment is a major concern to most countries. Give a reason for this. (1mk)
- Draw and name all possible isomers of C4H10 (3mks)
- The structure of a detergent is,
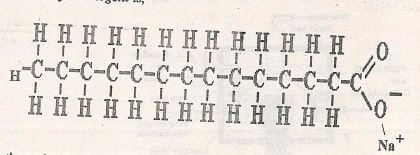
- Write the molecular formula of the detergent ....(1mk)
- What type of detergent is represented by the formula? (1 mk)
- When this type of detergent is used to wash linen in hard water, spots(marks) are left on the linen. Write the formula of the substance responsible for the spots. .....(1mk)
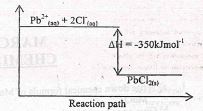
- Aqueous lead (II) nitrate react with aqueous Sodium chloride in a closed system forming a white precipitate of lead (II) chloride as shown by the equation below.
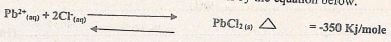
- State and explain the observation that would be made when;
- More Sodium chloride is added to the equilibrium mixture. . (1 mk)
- The temperature of the equilibrium mixture is raised. ........... (1 mk)
- Sketch an energy level diagram for the above reaction....(2marks)
- State and explain the observation that would be made when;
- Study the diagram below and then answer the questions that follow.
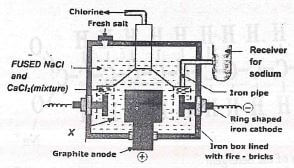
- Write down an ionic equation for the reaction taking place at the;
- Anode ..(1mk)
- Cathode (1mk)
- What is the importance of the part labelled X in the diagram. .....(1mk)
- Name one property that enables us to separate Sodium from sodium/calcium mixture....(1 mk)
- Write down an ionic equation for the reaction taking place at the;
- The equation given below represents a redox reaction.

- Write the equation for the reduction process (1mk)
- Which substance is the reducing agent? ...(1mk)
- The following diagram represents the blast furnace in which extraction of iron is carried out
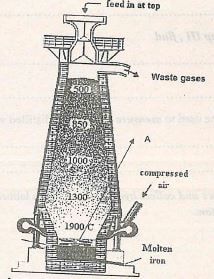
- Identify one other raw material used apart from the iron ore. (1mk)
- Write the equation that leads to the formation of substance A in the blast furnace. .................(1mk)
- State one property of the iron produced on the blast furnace. .............(1mk)
-
- State one way in which nuclear reactions differ from ordinary chemical reactions .. (1 mark)
- The following is a part of Uranium decay series.

- Which particles are emitted in Step 1
STEP I .. (1/2mk)
Step II.. (1/2mk) - If a beta particle is emitted in step III, find
Z and AZ.........(1/2mk)
......... (1/2mk)
- Which particles are emitted in Step 1
- Name two apparatus that can be used to measure 25.0cm3 of distilled water. ............... (1mk)
- Below is a set up used to prepare and collect hydrogen gas in the laboratory. Study it and then answer the questions that follow.
- Identify;
- Solid Q................(1mk)
- Apparatus R... (1mk)
- If the gas was to be collected dry, name a suitable drying agent that you would use to dry the gas. .....(1mk)
- Name any one industrial use of hydrogen gas. (1mk)
- Identify;

MARKING SCHEME
-
- Write down chemical formula of Manganese (IV) oxide
- MnO2
- Give one use of Manganese (IV) okide in the preparation of oxygen gas
- Catalyst
- Write down chemical formula of Manganese (IV) oxide
-
- Name two types of lames produced by a Bunsen bumer
- Non-luminous flame
- Luminous flame
- Describe how luminosity of a flame can be increased
- Opening the air hole
- Name two types of lames produced by a Bunsen bumer
-
- Define terms
- Lattice energy
- Is the energy change that occurs when one mole of an ionic substance is broken down into its constituent ions in their gaseous state
- Hydration energy
- Is the energy change that occurs when I mole of gaseous ions becomes hydrated.
- Lattice energy
- Given following find lattice energy of potassium bromide
△H soln = △H lattice + △H hydration
+8 = △Hlattice +(-655)
△H lattice +8 + 655
= +663kJmol-1
- Define terms
-
- State Boyles law
- The volume of a given mass of gas is inversely proportional to its pressure at constant temperature
- If pressure is raised to 1220mmHg, what volume would the gas occupy?
P1V1 = P2V2
650cm3 x 76mmHg = 1220mmHgV2
650cm3 × 760mmHg
1220mmHg
= 404.92cm3
- State Boyles law
-
- Select appropriate pH for each of the following
- Sour milk - 4 or 5
- Wood ash solution - 9
- Names gas Q
- Hydrogen chloride gas
- Which property of concentrated sulphuric (VI) acid is applied in the preparation for gas Q
- A less volatile acid
- Select appropriate pH for each of the following
- Determine mass of sodium hydroxide in 30.0cm3 of solution
- 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + H2O(l)
moles of H2SO4(aq) = 25 x 0.054moles
1000
= 0.00135 moles
Moles of NaOH = 0.00135 x 2 = 0.0027 moles
27cm3 of NaOH(aq) = 0.0027 moles
810cm3 = 810 x 0.0027
27
= 0.081 moles
Les of m 810cm3 = moles in 30cm3
RFM of NaOH = 40
1 mole of NaOH = 40
0.081 moles = 0.081 x 40 - 3.24g
- 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + H2O(l)
-
- What observations would be made if gas F is ignited?
- Bum with a pop sound
-
- Give a reason for this observation
- Iron is below magnesium in the reactivity series, thus it displaces less hydrogen from steam.
- What change in conditions of the experiment should the student have made in order to increase the volume of gas F produced
- Heat the iron powder.
- Give a reason for this observation
- What observations would be made if gas F is ignited?
- Calculate the percentage abundance of isotope 22Y
20(x + 82.1) + 21 x 0.26) + 22x
100
= 20.179
42x + 1647.46 - 2017.9
42x = 370.44
x=370.44
42
= 8 .82% - State and explain differences in melting point of
- Sodium and Aluminium
- Sodium has a lower melting point than aluminium sodium has one valence electron while aluminum has 3 (or fewer delocalized electrons than aluminium The strength of the metallic bond is weaker in sodium than in aluminium
- Oxygen and sulphur
- Sulphur has a higher melting point than oxygen. Sulphur exists as S, rings which has a higher relative molecular mass than 02. The melting point of sulphur is higher than that of oxygen.
(NB. the reverse is also true)
- Sulphur has a higher melting point than oxygen. Sulphur exists as S, rings which has a higher relative molecular mass than 02. The melting point of sulphur is higher than that of oxygen.
- Sodium and Aluminium
- Using dots (.) and crosses (x) diagram; show compound formed when aluminium Chloride is in vapour form
-
- Identify the pale blue precipitate
- Cu(OH)2
- Write an ionic equation for the formation of the deep blue solution
- Cu2+ (aq) + 4NH3(aq) → [Cu(NH3)4]2+(aq)
- Identify the pale blue precipitate
- Describe how each substance can be obtained from the mixture
- Heat the mixture to sublime ammonium chloride which forms on the cooler part of the apparatus used
- Add water to the mixture of copper (II) oxide and sodium chloride to dissolve sodium chloride
- Filer to get sodium chloride as the filtrate
- Evaporate the filtrate to get solid sodium chloride
- Copper oxide remains on the filter paper as the residue.
-
- State the observation that was made in the combustion tube at the end of the experiment
- Black copper (II)oxide changed to brown copper
- Write an equation for the reaction that took place in the combustion tube
- CO(g) + CuO(s) → Cu + CO2
- Why is it necessary to bum the gas coming out of the tube K?
- It is poisonous
- State the observation that was made in the combustion tube at the end of the experiment
-
- State the relationship between temperature and pressure that can be deduced from the graph
- Pressure is directly proportional to temperature or vice versa.
- using kinetic theory, explain the relationship shown on the graph
- As temperature increases the kinetic energy of the gaseous molecules increase. This increases the speed of the gascous molecules increasing the number of bombardness between the gaseous molecules and the walls of the container (pressure)
- State the relationship between temperature and pressure that can be deduced from the graph
-
- use one word to describe each of the changes in (1) and (ii)
- Deliquescent
- Efflorescent
- use one word to describe each of the changes in (1) and (ii)
-
- Name a reagent that can be used to distinguish between Al3+(aq) and Zn2+(ag) ions.
- Ammonia solution
- state what is observed if each ion is treated separately with the reagent
- Al3+(aq) forms a white precipitate with little ammonia solution. The white precipitate is insoluble in excess.
- Zn2+ forms a white precipitate with little ammonia. The white precipitate is soluble in excess
- Name a reagent that can be used to distinguish between Al3+(aq) and Zn2+(ag) ions.
-
- Give the chemical formula of gas Y
- N2O
- Give the confirmatory test for gas Y
- Relights a flowing splint
- State one use of gas Y
- Anaesthesia
- An oxidizer in racing car engines
- Flames for analytical work
- Give the chemical formula of gas Y
-
- Equation for reaction between hot concentrated sodium hydroxide and chlorine gas
- 6NaOH(aq) + 3Cl2(g) → NaClO3(aq)+ 5NaCl(aq) + 3H2O(l)
- Give any other difference between the two gases when it comes to bleaching action
- Cl2-bleaching is permanent
- SO2- Bleaching is temporary
- Give a reason for this
- I is pollutant/ Cl depletes the ozone layer/ it is poisonous forms acid-rain
NB: Accept any of the above.
- I is pollutant/ Cl depletes the ozone layer/ it is poisonous forms acid-rain
- Equation for reaction between hot concentrated sodium hydroxide and chlorine gas
- Draw and name all possible somers of C4H10
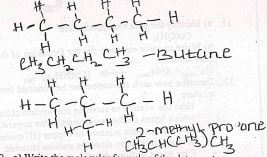
-
- Write the molecular formula of the detergent
- What type of detergent is represented by the formula?
- Soapy detergent
- Write formula of the substance responsible for the spots
- (CH3(CH2)2COO)2Mg or
- (CH3(CH2)2C00)2Ca
- Write the molecular formula of the detergent
- State and explain observation made when
- More sodium chloride is added to the equilibrium mixture
- White precipitate increases. Position of equilibrium shifts to the right to eliminate the increased CI.
- The temperature of the equilibrium mixture is raised
- The amount of white precipitate decreases. The position of equilibrium shifted to the left, to lower the temperature
- Sketch an energy level diagram for the above reaction
- More sodium chloride is added to the equilibrium mixture
-
- write down an ionic equation for reaction taking place at the
- Anode
2C1(g) → Cl2(g) + 2e- - Cathode
2Na+ +2e → 2Na(l)
- Anode
- What is the importance of the part labeled X in the diagram
- Diaphragm is used to prevent sodium and chlorine from mixing
- Name one property that enables us to separate sodium from sodium/ calcium mixture
- Density: Ca is denser than Na
- Melting point: Ca has a higher melting point than Na.
- write down an ionic equation for reaction taking place at the
-
- Write the equation for the reduction process
- 2H+(aq) + 2e- → H2(g)
- Which substance is the reducing agent?
- Mg(s)
- Write the equation for the reduction process
-
- Identify one other raw material used apart from the iron ore
- Limestone or coke
- Write the equation that leads to formation of substance A
- CaO(s) + SiO2(s) → CaSiO3(l) or CaO(s) + Al2O3(s) → CaAl2(l)
- State one property of the iron produced on the blast fumace
- Less hard (brittle)
- Identify one other raw material used apart from the iron ore
-
- State one way in which nuclear reactions differ from ordinary chemical reactions
encrry environmental factors such as temperatures or pressureNuclear Chemical Takes place in nucleus Takes place with valence e electrons Involve protons and neutrons Involve electrons Release large amount of energy Release or absorb little energy Not affected by Affected by environmental factors -
- Which particles are emitted in
Step I
4 (Alpha particles)
2
Step II
O ß (Beta particle)
-1 - If beta particle is emitted in step III, find Z and A
- Z-234
- A-92
- Which particles are emitted in
- State one way in which nuclear reactions differ from ordinary chemical reactions
- Name two apparatus that can be used to measure 25.0cm3 distilled water
- A pipette
- A burette
-
- Identify
- Solid Q
- Zinc metal
- Apparatus R
- Dropping funnel
- Solid Q
- Name suitable agent that you would use to dry the gas
- Anhydrous calcium chloride or
- Concentrated sulphuric (VI) acid c)
- Name any one industrial use of hydrogen gas
- Preparation of ammonia gas - Haber process
- Preparation of hydrochloric acid
- Manufacture of margarine (hydrogenation)
- Used in balloons (Meteorologists)
- As rocket fuel
- As a fiel in fuel cells
- Identify
Download Chemistry Paper 1 Questions and Answers - Sukellemo Joint Mock Examinations July 2020.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students