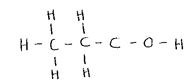Questions
Instructions to candidates.
- Answer ALL the questions in the spaces provided.
- ALL working must be clearly shown where necessary.
- Mathematical tables and silent electronic calculators may be used.
-
- Name the process that takes place when:
- Ethanol reacts with concentrated sulphuric (VI) acid at 180 o C to form ethene. (1 mark)
- Ammonium chloride is heated and forms a white powder on the cooler parts of the boiling tube. (1 mark)
- Propanol reacts with propanoic acid is the presence of a catalyst to form propyl propanoate. (1 mark)
- Give the systematic names of the following compounds : (2 marks)
- CH2CCH3
|
CH3 - CH3CH2CH2C≡CH
- CH2CCH3
- State the observation made when propan-l-ol reacts with:
- Acidified potassium dichromate (VI) solutions (1 mark)
- Sodium metal (1 mark)
- Ethanol obtained from glucose can be converted to ethane as shown below;
Step I Step II
C6H12O6 → C2H5OH → CH2=CH2
Name and describe the process that takes place in steps I and II (3 marks)
Step I
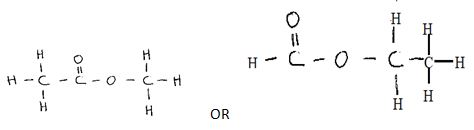
Step II - Compounds A and B have the same molecular formula C 3 H 6 O 2 . Compound A liberates carbon (IV) oxide on addition of aqueous sodium carbonate while compound B does not. Compound B has a sweet smell. Draw the possible structures of:
- Compounds A (1 mark)
- Compound B (1 mark)
- Give two reasons why the disposal of polymers such as polychloroethene by burning pollutes the environment. (2 marks)
- Name the process that takes place when:
-
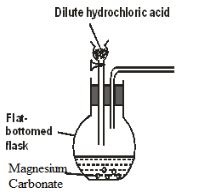
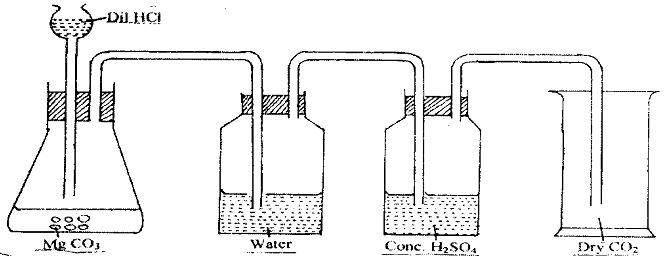
- The following diagram represents an incomplete set-up of apparatus used to prepare and collect a dry sample of carbon (IV) oxide gas.
- Complete the diagram to show how a dry sample of pure carbon (IV) oxide gas is collected. (2 marks)
- Write an equation for the reaction that takes place in the flat-bottomed flask. (1 mark)
- Why is it not suitable to use lead (II) carbonate in place of magnesium carbonate in this experiment? (1 mark)
- State two properties of carbon (IV) oxide that makes it suitable for use in fire extinguishers.(2 marks)
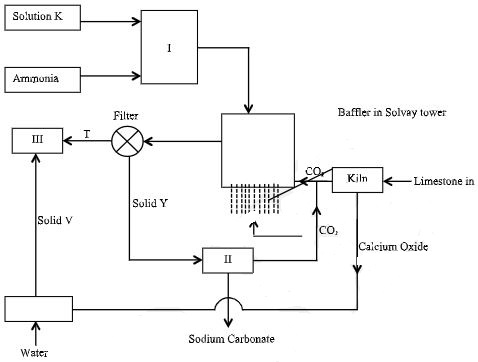
- Study the flowchart below then answer the questions that follow.
- Identify substances K, T, V and Y. (2 marks)
- Write an equation for the reaction that takes place in the Solvay tower. (1 mark)
- A burning magnesium ribbon will continue to burn in a gas jar of carbon IV oxide while a burning wooden splint will be extinguished. Explain. (3 marks)
- The following diagram represents an incomplete set-up of apparatus used to prepare and collect a dry sample of carbon (IV) oxide gas.
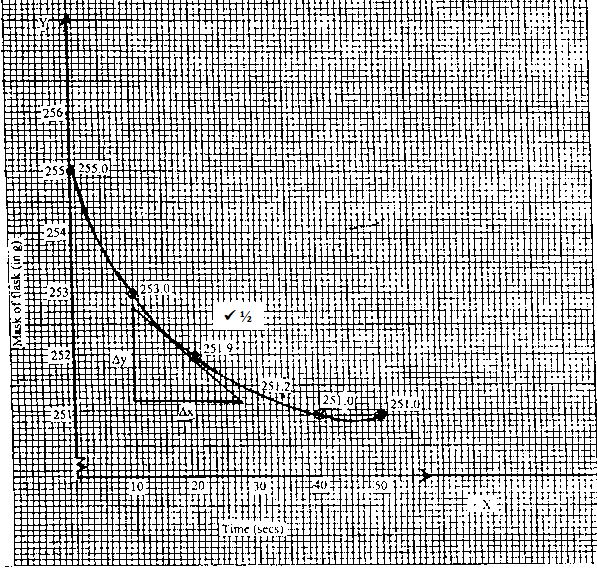
- 140g of powdered brass (an alloy of Zinc and copper) were added to excess 0.5M hydrochloric acid in a conical flask placed on top of a pan balance. The changes in mass of the flask and its contents with time were recorded in the following table. This experiment was carried out at room temperature.
Time (in seconds) 0 10 20 30 40 50 Mass(mg) of flask and its contents 255.0 253.0 251.9 251.2 251.0 251.0 - Write an equation for the reaction that took place. (1 mark)
- State and explain the relationship between the mass of the flask and its content with time. (2 marks)
- What observation was made in the flask at the end of the reaction? (1 mark)
-
- Plot a graph of mass of the flask and its content against time. (3 marks)
- Using the graph, determine rate of the reaction at the 20 th second. (2 marks)
- How would the rate in 4 (d) (ii) above be affected if the reaction was carried out using 0.5M hydrochloric acid at 45 o C? Explain. (2 marks)
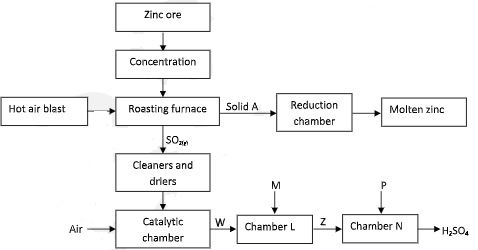
- The chart below represents the extraction of zinc from its ore and a by-product used in the manufacture of sulphuric (VI) acid. Study it and use it to answer the questions that follow:-
- Name;
- The zinc ore used. (1 mark)
- The main impurity in the ore. (1 mark)
-
- Name the process by which zinc ore is concentrated. (2 marks)
- State one use of Zinc metal (1 mark)
- Write an equation for the reaction taking place in the roasting furnace. (1 mark)
- Describe what happens in the reduction chamber. (2 marks)
- Identify substances:- (2 marks)
W
M - Write the equation for the reaction that occurs in chamber N. (1 mark)
- Explain why sulphur (VI) oxide is not dissolved directly in water. (2 marks)
- Explain the danger caused by this process to the environment. (2 marks)
- Name;
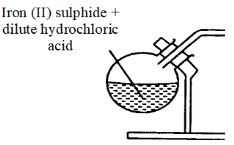
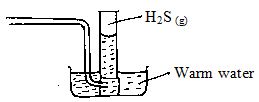
- Study the set up below which was used by form four students to prepare a gas in the laboratory.
- Complete the diagram to show how the gas above can be collected. (2 marks)
- Write the equation for the reaction in the flask. (1 mark)
- State a chemical test for the gas prepared. (1 mark)
- When hydrogen sulphide gas was bubbled into an aqueous solution of iron (II) chloride a yellow precipitate was deposited.
- State the other observation that was made. (1 mark)
- Write the reaction equation for d (i) above. (1 mark)
- When sugar crystals were reacted with concentrated sulphuric (IV) acid, a black substance T was formed which when dried burnt in excess air to form a colourless gas U only. While when concentrated sulphuric (VI) acid is reacted with liquid D at temperature of 170 o C, a colourless gas W is formed, this changes yellow bromine water to colourless and also changes colour of substance Y from purple to colourless.
- Identify;
Substance T (½ mark)
Gas U (½ mark)
Liquid D (½ mark)
Gas W (½ mark)
Substance Y…. (½ mark) - Which property of concentrated sulphuric (VI) acid is being demonstrated by formation of a black mass? (½ mark)
- Identify;
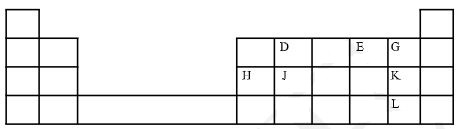
- The grid below is part of the periodic table. Use it to answer the questions that follow.
(The letters do not represent the actual symbols of the elements)- Which is the most reactive non-metallic element shown in the table? (1mark)
-
- Write the formula of the compound formed when element D and E react. (1 mark)
- Name the bond type in the compound formed in b (i) above. (1 mark)
-
- What is the name given to the group of elements where G, K and L belong? (1 mark)
- Write the equation for the reaction that occurs when G in gaseous form is passed through a solution containing ions of element L. (1 mark)
- The melting point of elements J and K are 1410 o C and -101 o C respectively. In terms of structure and bonding, explain why there is a large difference in the melting points of J and K. (2 marks)
- D forms two oxides. Write the formula of each of the two oxides. (2 marks)
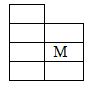
- M is an element that belongs to the 3 rd period of the periodic table and is a member of the alkaline earth metals. Show the position of M in the grid. (1 mark)
-
- The standard reduction potentials for five half cells are shown in the table below. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
E°(V) A2(aq) + 2e- → 2A-(aq) +1.09 Q2+(aq) + 2e- → Q(s) -0.13 R2+(aq) + 2e- → R(s) -2.37 Y2+(aq) + 2e- → Y(s) +0.34 - With a reason identify the strongest reducing agent. (1 mark)
- Write an equation for the cell formed when Q and Y half cells are joined. (1 mark)
- Calculate the e.m.f of the cell in (iii) above. (1 mark)
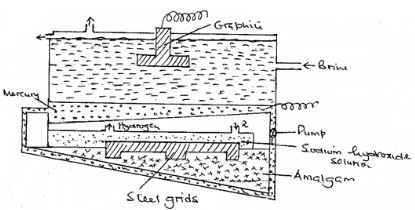
- The diagram below represents a mercury cell that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.
- Name raw material introduced at 2. (½ mark)
- Name another material that can be used in the cell instead of graphite. (½ mark)
- Write an equation for the reaction.
- that occurs at the anode. (1 mark)
- in which sodium hydroxide is produced. (1 mark)
- Give one reasons why mercury is recycled. (1 mark)
- A current of 100 amperes was passed through the cell for five (5) hours. Calculate the mass of sodium hydroxide that was produced. (3 marks)
(Na = 23.0, O = 16.0, H = 1.0, 1 Faraday = 96500C).
- The standard reduction potentials for five half cells are shown in the table below. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
Marking Scheme
-
-
- Dehydration
- Sublimation /Thermal decomposition
- Esterification
-
- 2-methylprop-1-ene /2-methylpropene
- Pent-1-yne
-
- Changes colour from orange to green
- Effervescence of a colourless gas that extinguish burning splint with a ‘pop’ sound is produced
- Step 1
Fermentation : Glucose is mixed with yeast and the enzyme in the yeast converts glucose to ethanol
Step II
Dehydration. Ethanol is heated with concentrated sulphuric (VI) acid or in presence of aluminium oxide (Al2O3) as a catalyst -
-
-
- Burning produces carbon (IV) oxide which causes global warming
It also produces acidic compounds which cause acid rain
-
-
-
-
-
- MgCO3(s) + HCI(aq) MgCI2(aq)+ H2O(l) + CO2(g)
- It will lead to formation of insoluble lead (II) sulphate which will coat the remaining lead (II) carbonate and the reaction will stop
-
- Denser than air
- Does not support combustion
-
-
-
- K – Brine (Concentrated NaCl solution)
- Y – Sodium hydrogen carbonate
- T – Ammonium chloride
- V – Calcium hydroxide
-
- NaCl(aq) + NH3(g) + CO2(g) + H2O(l) → NaHCO3(s) + NH4Cl(aq)
- Burning Magnesium produces a lot of heat that decomposes CO2 in to C and O2, O2, then support burning while heat from a burning wooden splint is not enough to decompose CO2
-
-
-
- Zn(s) + 2HCl(g) → ZnCl2(aq) + H2(g)
-
- The mass of the flask and its contents decrease as time increases until it comes to a constant mass
- When the reaction occurs, hydrogen gas is evolved and it escapes leading to loss of mass
- A brown solid (copper) remains at the bottom of the flask, No reaction took place with copper because it is lower than hydrogen in the reactivity series
-
-
- Draw a tangent of the curve at the 20th second as shown. Rate of reaction is the same as the gradient of the tangent at this point i.e
Reaction rate = Δy/Δx = 25.6 - 251.0
30 - 10
=1.6/20
= 0.08 g/s
Rate of reaction is 0.08g per second - The rate will be faster. AT higher temperature, the number of particles which will have attained activation increases and also since the kinetic energy of the particles has increased, there are more collisions per second hence the rate of reaction increases.
-
-
-
- Zinc blende
- Galena
-
- Froth floatation
- Used for coating of iron objects/galvanizing
Used for making the casing of dry cells which act as negative electrode
Used to make alloys such as brass
- 2ZnS(S) + 3O2(g) → 2ZnO(S) + 2SO2(g)
- Zinc oxide is reduced by both carbon and carbon II oxide to zinc vapour.
- W – SO3/Sulphur VI oxide
M – Concentrated sulphuric VI acid or conc H2SO4 - H2S2O7(l) + H2O(l) → 2H2SO4(aq)
- The process is highly exothermic The heat produced boils the acid leading to vapour droplet which are difficult to condense. (acid spray/mist of acid)
- SO2 dissolves in water vapour/moisture to form acid droplets which corrodes buildings and affects aquatic life.
-
-
-
- FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g)
- Blackens wet lead ethanoate powder/Forms a black precipitate of PbS with lead(II)nitrate solution
Any other possible test -
- A green solution is formed
- 2FeCl3(aq) + H2S(s) → 2FeCl2(aq) + S(s) + 2HCl(aq)
-
- T – Carbon
U – Carbon (IV) oxide
D – Ethanol
W – Ethene
Y – Acidified potassium manganate (VII) - Dehydration
- T – Carbon
-
-
- Element G. It readily gains an electron to attain stability/Highest -ve Eθ value
-
- DE2
- Covalent bond
-
- Halogens
- G2(g) + 2L-(aq) → 2G-(aq) + L2(s)
- Atoms of J are bonded by strong covalent bonds forming a giant atomic structure hence high boiling point unlike molecules of K which are held by weak van der Waal’s forces forming simple molecular structure.
- DO and DO2
-
-
-
- R(s) - Has the highest negative Eθ value // loses electrons most readily.
- Y2+(aq) + Q(s) → Y(s) + Q2+(aq)
- Ecell = +0.34 + 0.13 = +0.47V
-
- Water Accept distilled water or H2O
- Platinum // Titanium
-
- Anode → 2Cl-(aq) → Cl2(g) + 2e
- 2NaHg(l) + H2O(l) → 2NaOH(aq) + 2Hg(l) + H2(g)
OR
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(l)
-
- Reduce costs// its economical // Hg is expensive.
- Reduce environmental pollution // Hg is poisonous.
- Q = It = 100 x 5 x 60 x 60 = 1800000C
2Na+(aq) + 2e- → 2Na
Na+ + e- + Hg → NaHg
IF ≡ 1 mol Na ≡ 1 mol NaoH
96500C → 40g NaOH
1800000C → d
d = 40 x 1800000 = 746.1g
96500
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Bondo Joint Mocks Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students