Instructions to Candidates
- All working MUST be clearly shown where necessary
- Candidates should answer the questions in English

QUESTIONS
- State TWO reasons why most apparatus are made of glass (2marks )
- A pipette is used to measure exact volume of liquids. Draw a pipette. (1 marks)
- An atom exists as an isotope X-30, X-29 and X-33 has relative atomic mass of 30.30, if X-30 is 10% calculate the percentage abundance of the other two isotopes (2marks)
- The diagram below shows electrolysis of lead (II) bromide
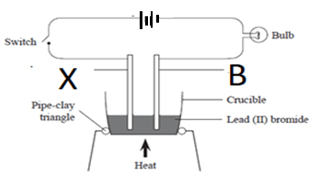
- Name electrode B (1 mark)
- Explain the observation made in electrode X (1 mark)
- About 40cm3 of oxygen gas were reacted with 100cm3 of hydrogen gas.
- Determine the volume of residual gas at 105°C (3 marks )
- What volume of oxygen was used during the reaction? (1 mark)
- Ethene gas can be prepared as follows
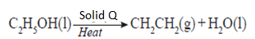
- Name solid Q (1 mark)
- Give two functions of solid Q in the process (1 mark)
- Name the following organic compound (1 mark)
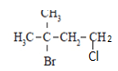
-
- Define the term fractional crystallisation (1 mark )
- A salt solution has a mass of 65g containing 5g of solute. The solubility of this salt is 25g per 100g water at 20°C. 60g of the salt are added to the solution at 20°C. Calculate the mass of the solute that remain undissolved (2 marks)
- A sample of river water was divided into three portions. The table below shows the test carried out on the portions and the observations made
.Test Observation Interference To he first portion, 1cm3 of soap solution was added No lather formed he second portion was boiled, cooled and 1 cm3 of soap solution was added. No lather formed o the third porion, 3cm3of aqueous sodium carbonate was added, the mixure filtered and 1cm3 of soap solution added to filtrate Lather formed immediately
Complete the table by filling in the inferences (3 marks) - The following diagram represents extraction of sodium by the Down’s cell
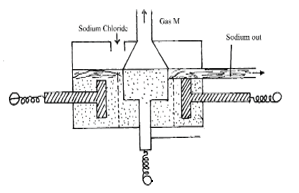
- What is the function of heat resistant wall (1 mark)
- Why is the anode made of graphite? (1 mark)
- How are the electrolytic products separated from reacting? (1 mark)
- Explain why it is possible to separate components at the cathode (1 mark)
- Describe how a mixture of sodium chloride and lead chloride can be separated in the laboratory. (3marks)
- Study the diagram below and answer the following questions.
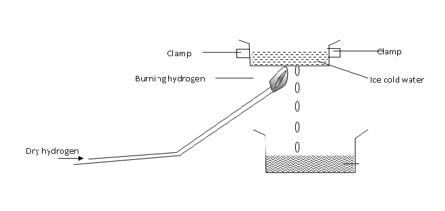
- Write equation that produce the flame in the experiment. (1 mark)
- What is the aim of the experiment? (1 mark)
- Form 2 students arranged the set up below to study some properties of heating Lead(II) nitrate strongly.
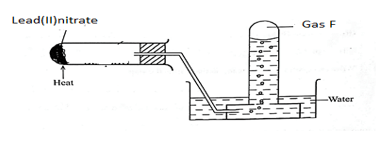
- State the observation during the experiment. (1 mark)
- Write down a balanced chemical equation for the reaction (1 mark)
- Describe a chemical test for gas F (2 marks)
- Distinguish a dry salt from anhydrous salt. (1 mark)
- 2.56g of hydrocarbon contains carbon to hydrogen in the ratio of 5.1. If the molecular mass is 128. Calculate the molecular formula of the hydrocarbon (3 marks) (C=12, H=1)
- Chlorine water is a bleaching agent. Describe the bleaching action of chlorine using relevant equation. (3marks)
- During electrolysis of dilute Magnesium Sulphate, using inert electrode. Explain
- The effect on concentration of the electrolyte during electrolysis. (2 marks)
- The difference in volume of the gasses produced at each electrode. (2 marks)
- The diagram below shows a ‘jiko’ when in use. Study it and answer the questions that follow
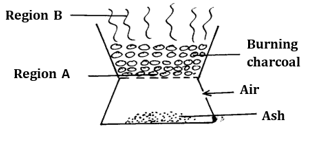
- Identify the gas formed at region B (1mark)
- Using an equation, explain what happens at region A (2 marks)
-
- What is half- life? (1mark)
- The half-life of protactinium - 234 is 1.17 minutes. Determine the mass that decays in 5.85 minutes starting with 100 g of the sample. (2 marks)
- Given the following substances: sodium carbonate, orange juice and sodium bromide.
- Name one commercial indicator that can be used to show whether sodium carbonate, orange juice and sodium bromide are acidic, basic or neutral. (1mark)
- Classify the substances in 15 (a) above as acids, bases or neutral. (2 marks)
Acid Base Neutral
- A reaction is described as “having reached equilibrium”. What does this statement mean regarding the amounts of the reactants and products? (1 mark)
- Suggest two ways in which the equilibrium concentration of SO3(g) can be increased in a closed container, if the only chemical equilibrium is; (2 marks)
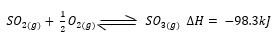
- Industrial process of production of NO(g) is represented by reaction:
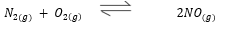
The reaction is carried out at elevated temperatures to drive the reaction towards the formation of the product. After sufficient products has formed the reaction mixture is quickly cooled. Explain. (2 marks) - The Diagram below may be used to react hydrogen sulphide and Sulphur (IV) oxide. Study it and answer the questions that follow.
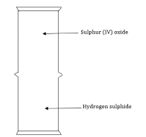
- What is observed in the jars? (1 mark)
- Write an equation for the reaction. (1 mark)
- What is the role of Sulphur (IV) oxide in the reaction? (1 mark)
- Element A atomic no. 6 and element B atomic no. 13 react to form a compound.
- Using dots (•) and crosses (×) show bond formed in the above compound. (1 mark)
- Explain why the compound above has very high melting point. (1 mark)
- Explain how the compound above will conduct electricity. (1 mark)
- The diagram below shows relationship of basic and acidic oxides. In which region will compound of A and B fall? Explain. (1 mark)
- 100cm3 of gas X takes 30 seconds to diffuse through a porous plug, whereas 300cm3 of oxygen gas takes 120 seconds. Calculate the relative molecular mass of gas X (O=16) (3 marks)
- Ammonium nitrate was heated as shown below
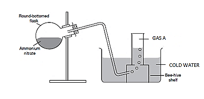
- Identify gas A (1 mark)
- Identify a mistake in the set up and give a reason (2 marks)
- Gas A was passed over heated copper in a combustion tube, state the observation made (1mark)
-
- State Hess’s law (1 mark)
- Given the following energies
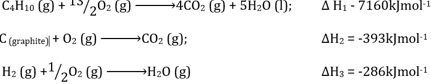
Draw energy circle diagram linking of formation of butane to its heat of combustion and heat of combustion and heat of combustion of carbon and hydrogen (2 marks) - Use the energy circle to calculate the heat of formation of butane (2 marks)
-
- What is a fuel (1mark)
- State two factors to consider when choosing fuel ( 2 marks )

Marking Scheme
- State TWO reasons why most apparatus are made of glass (2marks )
- easy to clean
- Transparent one ccan see through it
- Most chemicals do not react with glass
- A pipette is used to measure exact volume of liquids. Draw a pipette. (1 marks)
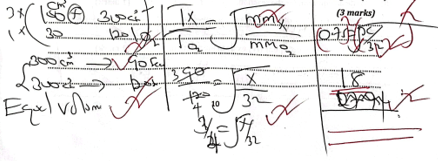
- An atom exists as an isotope X-30, X-29 and X-33 has relative atomic mass of 30.30, if X-30 is 10% calculate the percentage abundance of the other two isotopes (2marks)
- (30*10)+29(x)+33(90-x)/100 =30.30
300+29x+2970+33x=3030
3270-4x=3030
4x=-240
x=60
- (30*10)+29(x)+33(90-x)/100 =30.30
- The diagram below shows electrolysis of lead (II) bromide

- Name electrode B (1 mark)
- cathode
- Explain the observation made in electrode X (1 mark)
- Blown gas,form Br oxidixed to Br2(g)
- Name electrode B (1 mark)
- About 40cm3 of oxygen gas were reacted with 100cm3 of hydrogen gas.
- Determine the volume of residual gas at 105°C (3 marks )
- 2H2(g)+O2(g)→2H2O(g)
H2=20 100/(80) (40)
20+80=100CM3
- 2H2(g)+O2(g)→2H2O(g)
- What volume of oxygen was used during the reaction? (1 mark)
- Determine the volume of residual gas at 105°C (3 marks )
- Ethene gas can be prepared as follows
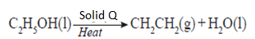
- Name solid Q (1 mark)
- aluminum oxide
- Give two functions of solid Q in the process (1 mark)
- dehydrating agent
- its a catalyst
- Name the following organic compound (1 mark)
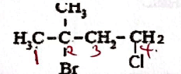
- Name solid Q (1 mark)
-
- Define the term fractional crystallisation (1 mark )
- process of seperaing solution with difference in solubilities
- A salt solution has a mass of 65g containing 5g of solute. The solubility of this salt is 25g per 100g water at 20°C. 60g of the salt are added to the solution at 20°C. Calculate the mass of the solute that remain undissolved (2 marks)
- 65-25=40g
40g+5=45
65-60=5
(60-25)=45+5 =50g
- 65-25=40g
- Define the term fractional crystallisation (1 mark )
- A sample of river water was divided into three portions. The table below shows the test carried out on the portions and the observations made
.Test Observation Interference To he first portion, 1cm3 of soap solution was added No lather formed permanent waer harder he second portion was boiled, cooled and 1 cm3 of soap solution was added. No lather formed permanent water harder o the third porion, 3cm3of aqueous sodium carbonate was added, the mixure filtered and 1cm3 of soap solution added to filtrate Lather formed immediately temporary waer harder
Complete the table by filling in the inferences (3 marks) - The following diagram represents extraction of sodium by the Down’s cell

- What is the function of heat resistant wall (1 mark)
- maintain high temperature and conserve heat
- Why is the anode made of graphite? (1 mark)
- carbon does not react with chlorine
- How are the electrolytic products separated from reacting? (1 mark)
- using steel diaphragm
- Explain why it is possible to separate components at the cathode (1 mark)
- Calcium is denser and easily crystalised leaving behind NA in molen form
- What is the function of heat resistant wall (1 mark)
- Describe how a mixture of sodium chloride and lead chloride can be separated in the laboratory. (3marks)
- Add cold water and stir NCCL disolve filtrate to obtain Pbcl2 as residue, Heat Nacl solution to satration
- Study the diagram below and answer the following questions.

- Write equation that produce the flame in the experiment. (1 mark)
- 2H2(g)+O2(g)→2H2O(g)
- What is the aim of the experiment? (1 mark)
- steam condense to water when cooled
- condensation of gas to liquid
- Write equation that produce the flame in the experiment. (1 mark)
- Form 2 students arranged the set up below to study some properties of heating Lead(II) nitrate strongly.

- State the observation during the experiment. (1 mark)
- Brown fumes Orange residue
- Write down a balanced chemical equation for the reaction (1 mark)
- 2Pb(NO3)2→2PbO(s)+4NO2(g)+O2(g)
- Describe a chemical test for gas F (2 marks)
- State the observation during the experiment. (1 mark)
- Distinguish a dry salt from anhydrous salt. (1 mark)
- A dry salt is a salt without water while anhydrous salt is a salt that has had the waer driven out
- 2.56g of hydrocarbon contains carbon to hydrogen in the ratio of 5.1. If the molecular mass is 128. Calculate the molecular formula of the hydrocarbon (3 marks) (C=12, H=1)
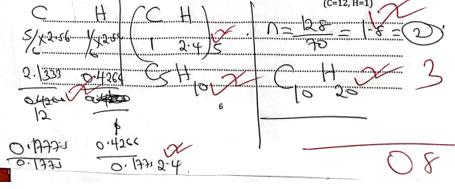
- Chlorine water is a bleaching agent. Describe the bleaching action of chlorine using relevant equation. (3marks)

- During electrolysis of dilute Magnesium Sulphate, using inert electrode. Explain
- The effect on concentration of the electrolyte during electrolysis. (2 marks)
- Mg2+,SO2- concentrate increases as water is a lot during discharge of H+ and OH-
- The difference in volume of the gasses produced at each electrode. (2 marks)
- Volume of H2 twice Volume of O2
- The effect on concentration of the electrolyte during electrolysis. (2 marks)
- The diagram below shows a ‘jiko’ when in use. Study it and answer the questions that follow

- Identify the gas formed at region B (1mark)
- carbon (IV) oxide
- Using an equation, explain what happens at region A (2 marks)
- C(s)+O2(g)→CO2(g)
carbon undergoes full combustion forming CO2
- C(s)+O2(g)→CO2(g)
- Identify the gas formed at region B (1mark)
-
- What is half- life? (1mark)
- Time taken for a given mass of radioactive isotope to decay to half it's original mass
- The half-life of protactinium - 234 is 1.17 minutes. Determine the mass that decays in 5.85 minutes starting with 100 g of the sample. (2 marks)
- 5.58/1.17
100→50→25→12.5→6.25→3.125
5 half-life
- 5.58/1.17
- What is half- life? (1mark)
- Given the following substances: sodium carbonate, orange juice and sodium bromide.
- Name one commercial indicator that can be used to show whether sodium carbonate, orange juice and sodium bromide are acidic, basic or neutral. (1mark)
- methyl orange, phenolphthalein
- Classify the substances in 15 (a) above as acids, bases or neutral. (2 marks)
Acid orange juice Base sodium carbonate Neutral sodium Bromide
- Name one commercial indicator that can be used to show whether sodium carbonate, orange juice and sodium bromide are acidic, basic or neutral. (1mark)
- A reaction is described as “having reached equilibrium”. What does this statement mean regarding the amounts of the reactants and products? (1 mark)
- Suggest two ways in which the equilibrium concentration of SO3(g) can be increased in a closed container, if the only chemical equilibrium is; (2 marks)
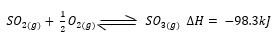
- insrease pressure
- decrease temperature
- Industrial process of production of NO(g) is represented by reaction:
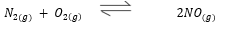
The reaction is carried out at elevated temperatures to drive the reaction towards the formation of the product. After sufficient products has formed the reaction mixture is quickly cooled. Explain. (2 marks)- to decrease temperature
- to increase pressure
- The Diagram below may be used to react hydrogen sulphide and Sulphur (IV) oxide. Study it and answer the questions that follow.

- What is observed in the jars? (1 mark)
- yellow deposits
- Write an equation for the reaction. (1 mark)
- SO2+2H2S→3S+2H2O
- What is the role of Sulphur (IV) oxide in the reaction? (1 mark)
- Oxidising agent H2S to S
- What is observed in the jars? (1 mark)
- Element A atomic no. 6 and element B atomic no. 13 react to form a compound.
- Using dots (•) and crosses (×) show bond formed in the above compound. (1 mark)
- Explain why the compound above has very high melting point. (1 mark)
- strong ionic bond and giant ionic structure
- Explain how the compound above will conduct electricity. (1 mark)
- molten state- free mobile ions
- The diagram below shows relationship of basic and acidic oxides. In which region will compound of A and B fall? Explain. (1 mark)

- Amphoteric oxide both basic and acid properties
- Using dots (•) and crosses (×) show bond formed in the above compound. (1 mark)
- 100cm3 of gas X takes 30 seconds to diffuse through a porous plug, whereas 300cm3 of oxygen gas takes 120 seconds. Calculate the relative molecular mass of gas X (O=16) (3 marks)
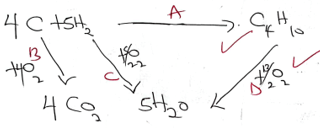
- Ammonium nitrate was heated as shown below

- Identify gas A (1 mark)
- Nitrogen(I)Oxide
- Identify a mistake in the set up and give a reason (2 marks)
- collecting N2O over cold water
- Heat is missing- NH4NO3 will not develop
- Gas A was passed over heated copper in a combustion tube, state the observation made (1mark)
- Brown copper melted changed o black copper(II)Oxide
- Identify gas A (1 mark)
-
- State Hess’s law (1 mark)
- a chemical reaction is independent of the rou of chemical reacions while keeping the same initail and final conditions
- Given the following energies
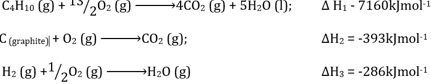
Draw energy circle diagram linking of formation of butane to its heat of combustion and heat of combustion and heat of combustion of carbon and hydrogen (2 marks) - Use the energy circle to calculate the heat of formation of butane (2 marks)
- A=4B+5C-D
A=4(-393)+5(-286)-(+160)
+4158kJ/mol
- A=4B+5C-D
- State Hess’s law (1 mark)
-
- What is a fuel (1mark)
- A substance that produce useful energy when it undergoes combustion
- State two factors to consider when choosing fuel ( 2 marks )
- cost
- Availability
- Haeting value
- Eve of combustion
- What is a fuel (1mark)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Kassu Jet Joint Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students