INSTRUCTIONS
- Answer all the questions in English
- Mathematical tables and electronic calculators may be used
- All working must be clearly shown where necessary

QUESTIONS
-
- The graph below represents the trend in melting points of elements in period 3.
Study it and use it to answer the questions that follow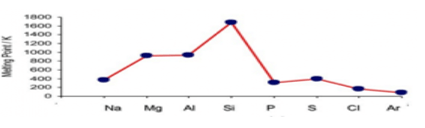
- Explain the trend in melting point between Aluminum and Phosphorous. (2 marks)
- Give a reason why Argon has the lowest melting point (1 mark)
- The table below shows the properties of several elements. Study it and use it to answer the questions that follow.
Element
Atomic radius (nm)
Ionic radius (nm)
P
0.136
0.065
Q
0.174
0.099
R
0.099
0.181
S
0.203
0.133
- Giving a reason, identify the non metal (2 marks)
- Given that, element P and S belong to the same period of the periodic table, identify the element with a lower ionization energy. Explain. (2 marks)
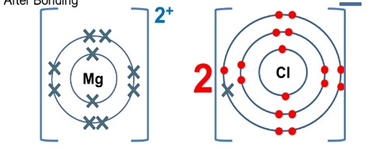
- An element X forms an ion with the formula X2+. The electronic configuration of the ion is 2.8
- State the group and period to which element X belongs. (1mark)
Group ………………………………..……………….
Period ………………………………………………… - Draw dot and cross diagram showing bonding when X combines with chlorine (1 mark)
- State the group and period to which element X belongs. (1mark)
- Explain the following observations;
- Carbon has more than one melting point (1 mark)
- Silicon and phosphorous are in the same period but at room temperature, the oxide of silicon is a solid, while the oxide of sulphur is gaseous (1 mark)
- The graph below represents the trend in melting points of elements in period 3.
-
- Determine the oxidation state of the element indicated in brackets (3 marks)
- MnO4 – (Mn)
- K2 Cr2 O7 (Cr)
- H3PO4 (P)
- Below is a list of standard reduction potentials of some elements. Use it to answer the questions that follow.
A 2+ (aq) + 2e – → A (s) + 0.34 V
N 2+ (aq) + 2e – → N (s) – 0.76 V
G + (aq) + 2e – → ½ G (s) 0.00 V
Y 2+ (aq) + 2e – → Y (s) + 0.88 V
L 2+ (aq) + 2e – → L (s) – 2.16 V- Identify the strongest reducing agent (1 mark)
- Explain why a solution containing A2+ ions cannot be stored in a container made of metal N (1 mark)
- The half cells of Y and L were combined to form an electrochemical cell.
- Draw a well labelled diagram of the cell formed (3 marks)
- Calculate the e.m.f of the cell formed above (1 mark)
- The diagram below shows the set up used to investigate electrolysis of dilute sulphuric (VI) acid solution
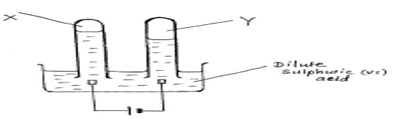
- Identify product X and Y (1 mark)
X ……………………………………
Y …………………………………… - Write an equation for the reaction at the anode (1 mark)
- Explain what happens to the solution after 2 hrs sometime (1 mark)
- Identify product X and Y (1 mark)
- Determine the oxidation state of the element indicated in brackets (3 marks)
- Study the diagram below and use it to answer the questions that follow
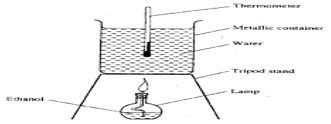
- During the experiment, the following data was collected
Volume of water = 400 cm3
Initial temperature of water = 23.0°C
Final temperature of water = 35.0°C
Initial mass of lamp and ethanol = 99.07 g
Final mass of lamp and ethanol = 98.23 g
Specific heat capacity = 4.2 kJ Kg-1 K-
Calculate the;- Temperature change (1 mark)
- Heat change for the reaction (2 marks)
- Mass of ethanol that reacted (1 mark)
- Molar enthalpy of combustion of ethanol (C=12, H=1.0, O=16.0) (2 marks)
- Use the information in the table below to answer the questions that follow
Na+ (g) + Cl- (g) → NaCl (s) ∆H1 = – 776 kJ/ Mol
Na+ (g) + aq → Na+ (aq) ∆H2 = – 390 kJ/ Mol
Cl- (g) + aq → Cl- (aq) ∆H3 = – 384 kJ/ Mol- Give the name of (2 marks)
∆H1 ……………………………………………………………………..
∆H3 …………………………………………………………………….. - Using an energy cycle diagram, calculate the molar enthalpy of solution of sodium chloride (3 marks)
- Give the name of (2 marks)
- During the experiment, the following data was collected
- Study the flow diagram below and use it to answer the questions that follow
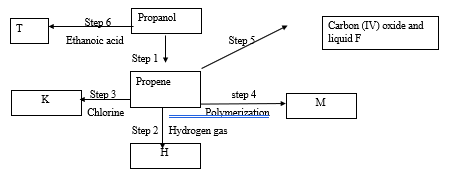
- Name substance; (3 marks)
- H …………………………………………………………………
- T ……………………………………………………………………
- F ……………………………………………………………………
- State the conditions for (2 marks)
- Step 1
- Step 2
- Write an equation for the reaction in (2 marks)
- Step 6
- Step 5
- Draw the structural formula of substance M (1 mark)
- A sample of substance M was found to have a molar mass of 47,208. Calculate the number of monomers in the sample. (2 marks)
- Name the process taking place in step 1 (1 mark)
- Identify the reagent used in step 5 (1 mark)
- Name substance; (3 marks)
-
- Define the following terms
- radioactivity (1 mark)
- Define half-life (1 mark)
- In an experiment to determine the half life of Radon – 220, the following results were obtained.
Time (seconds)
0
10
20
30
40
50
60
70
Count rate per second
30
26
23
21
18
16
14
12
- On the grid provided, draw a graph of count rate against time (3 marks)
- from the graph, determine the half-life of radon – 220 (1 mark)
- State one application of radioactivity in; (2 marks)
Agriculture
Medicine
- The diagram below shows the radiations emitted by a radioactive sample.
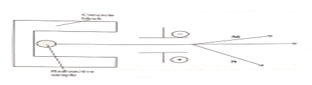
- Identify radiation (2 marks)
M ……………………………………………….
N ……………………………………………… - Explain the difference in the deflection of M and N (1 mark)
- Identify radiation (2 marks)
- Define the following terms
- The flow chart below shows the process of extraction of zinc. Study it and answer the questions that follow.
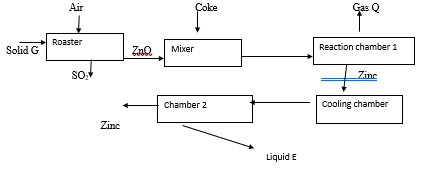
- Identify (1 mark)
- Solid G ……………………………………………………………………
- Gas Q …………………………………………………………………
- Name the other substance introduced into the mixer and state its role (2 marks)
- write an equation for the reaction in; (2 marks)
- Roaster
- Reaction chamber 1
- Describe the process that takes place in the cooling chamber (2 marks)
- Name the main impurity found in the zinc ore (1 mark)
- Explain one danger caused by this process (1 mark)
- A student found a piece of metal that he suspected could be zinc (II) ions. Describe three successive tests he would carry out to confirm the solid is zinc and give the observations expected in each test. (3marks)
Test
Procedure
Expected observation
1
2
3
- Identify (1 mark)
- The diagram below shows the laboratory preparation of ammonia gas. Study it and use it to answer the questions that follow.
- Name the reactants used (1 mark)
- Give the role of calcium oxide (1 mark)
- State 2 physical properties of ammonia gas (1 mark)
- Write an equation for the reaction (1 mark)
- Ammonia reacts with oxygen in the presence of a catalyst to produce nitric (V) acid industrially.
- Name the catalyst used (1 mark)
- Describe how the product in e above is converted to nitric (V) acid (2 marks)
- Ammonia and sulphuric (VI) acid are reacted to form a fertilizer.
- Write an equation for the reaction. (1 mark)
- Calculate the volume of ammonia required at STP to manufacture 1500kg of the fertilizer at STP (N= 14.0, H= 1.0, S= 32, O= 16.0, MGV at STP = 22.4L) (3 marks)

MARKING SCHEME
-
-
- aluminium has strong metallic bonds. Silicon has stronger covalent bond hence melting point increases. Phosphorous has weak van der waals forces hence the melting point drops///
OR
aluminium has giant metallic structure, silicon has giant covalent structure while phosphorous has molecular structure - argon is a monoatomic gas with the weakest van der waals forces
- aluminium has strong metallic bonds. Silicon has stronger covalent bond hence melting point increases. Phosphorous has weak van der waals forces hence the melting point drops///
-
- R, its ionic radius is greater than its atomic radius hence reacts by gaining an electron
- S, it has a bigger atomic radius hence requires less energy to remove an electron from the outermost energy level
-
- group II period 3
-
-
- carbon has allotropes which have different melting points
- Oxide of silicon has covalent bonds in its giant covalent structure while the oxide of sulphur is molecular with weak van der waals forces between molecules
-
-
-
- x + 4(-2) = -1, x = +7
- 2(1) + 2(x) + 7(-2) = 0, x = +6
- 3 + x + 4(-2) = 0 x = +5
-
- L
- Metal N will react with the ions of A OR metal L will displace the ions of A
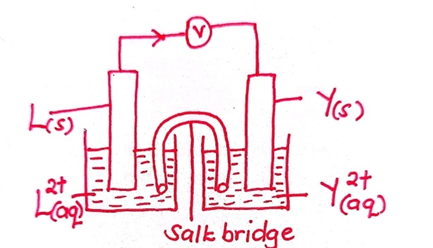
- +0.88 – (-2.16) = +3.04V
-
- X – oxygen gas Y – Hydrogen gas
- 4OH- (aq) → 4e + 2H2O (l) + O2 (g)
- solution becomes concentrated as water is removed OR the ions of water are discharged
-
-
-
- 35 – 23 = 120 C
- 400/1000 x 4.2 x 12 = 20.16 kJ
- 99.07 – 98.23 = 0.84g
- (46 x 20.16)/ 0.84 = 1104 kJ/ mol
-
- ∆H1 lattice enrgy
∆H3 hydration energy of chlorine -
776 + (- 390 – 384) = +2 kJ/Mol
- ∆H1 lattice enrgy
-
-
-
- propane
- propylethanoate
- water
-
- conc sulphuric (VI) acid and 170 – 180 0
- 1500 C , Pt/ Pd catalyst
-
- CH3 COOH (aq) + CH3CH2CH2OH → CH3 COO CH2CH2CH3 (aq) + H2O (l)
- 2C3H6 (g) + 9O2 (g) → 6CO2 (l) + 6H2O (l)
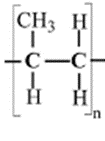
- (3(12) + 6 )n = 47,208
n = 1124 - dehydration
- oxygen gas OR air
-
-
-
- It is the spontaneous disintegration of a radioactive substance with emission of radiations and energy
- time taken for a radioactive nuclide to decay to half its original mass
-
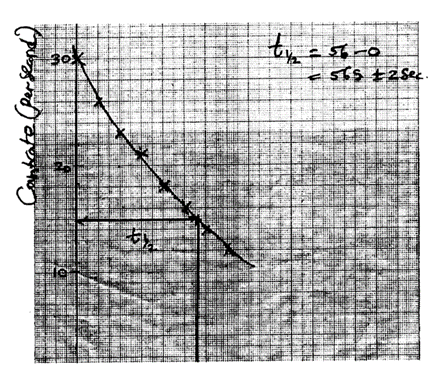
- 56 seconds
- agriculture
monitor rate of photosynthesis
monitor absorption of phosphate fertilizers
medicine
sterilizing medical instruments
imaging
heart pacesetters
treatment of cancer
detecting goitre and ulcers
-
- M alpha
N beta - beta particles have a very small mass hence have a greater deflection OR M is positively charged while N is negatively charged
- M alpha
-
-
-
- Zinc Sulphide OR Zinc blende
- Carbon (II) oxide or Carbon (IV) oxide (accept correct formula )
- Calcium carbonate or limestone
to produce carbon (IV) oxide that is used to reduce carbon/// to provide calcium oxide that is used to remove impurities -
- 2ZnS (s) + 2O2 (g) → 2ZnO (s) + SO2(g)
- ZnO (s ) + CO (g) → Zn (s) + CO2 (g) OR
ZnO (s) + C (s) → Zn (s) + CO2 (g)
- The mixture is sprayed rapidly by molten lead to cool at 6000 C. the molten zinc separated and settles on top of the molten lead.
- Lead (II) sulphide
- The gaseous products lead to pollution
open pits left after mining degrade the land
-
|
Test |
Procedure |
Expected observation |
|
1 |
Add hydrochloric acid or any suitable acid to the sample and divide into 2 portions ( ½ mark) |
effervescence (½ mark) |
|
2 |
To the first portion add sodium hydroxide dropwise until in excess (½ mark) |
White ppt which dissolves (½ mark) |
|
3 |
To the second portion add aqueous ammonia dropwise until in excess (½ mark) |
White ppt which dissolves (½ mark) |
Accept heating strongly, followed by addition of an acid and finally addition of ammonia in excess.
-
- ammonium chloride and calcium hydroxide or any other appropriate ammonium salt
- to dry the gas
- soluble in water
alkaline
chocking smell
less dense than air - NH4Cl (s) + Ca(OH)2 (s) → CaCl2 (s) + NH3 (g) + H2O (l)
-
- platinum
nitrogen (II) oxide is reacted with air to form nitrogen (IV) oxide. Water is added to the mixture to form nitric (V) and nitric (III) acids. The mixture is heated to distill off nitric (V) acid.
- platinum
-
- NH3(g) + H 2SO 4 (aq) + (NH4)2SO 4 (aq)
- moles ammonium sulphate = 1500/132 = 11.36 moles
volume = 11.36x 22.4 = 254.54 litres
Download Chemistry Paper 2 Questions and Answers - Nginda Girls Mock Examination 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
