Instructions to Candidates
- Answer ALL the questions
- KNEC Mathematical tables and silent non-programmable electronic calculators may be used.
- All working MUST be clearly shown where necessary
- Candidates must answer all the questions in English.
-
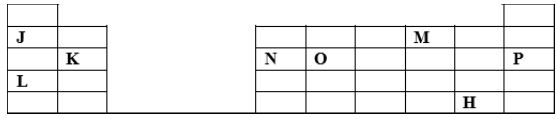
- The grid below represents a periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements
- Write the formula of the compound formed by element J and M. (1mk)
- Identify the least reactive element. Give a reason for your answer. (1mk)
- Compare the atomic size of K and O. Explain. (2mks)
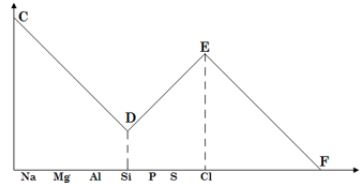
- The following graph shows the reactivity of elements in period 3.
Explain the Trend at D and E (1mks) - The table below gives information on the Melting Points of compounds of period 3 elements. The letters do not represent the actual symbols.
- Write the formula of
Elements R S T U V W Atomic number 11 12 13 14 15 16 M.pt of chloride °C 80` 714 − −70 −90 − 80 M.pt of oxide °C 1190 3080 2050 2750 560 −73 - Chloride of T (1mk)
- Oxide of U (1mk)
-
- Using the information above, suggest the type of bonding present in the chloride of V. Explain. (2mks)
- The difference in melting point of chloride and oxide of U in terms of structure and bonding. (2mks)
- Why there is no melting point in the chloride of T. (1mk)
- Write the formula of
- The grid below represents a periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements
-
- Give the systematic names of the compounds whose structural formulae are given below.
-
(½ mark)
-
(½ mark)
-
- Draw and name the structural formulae of the compound obtained when compounds in (a) react. (1 mark)
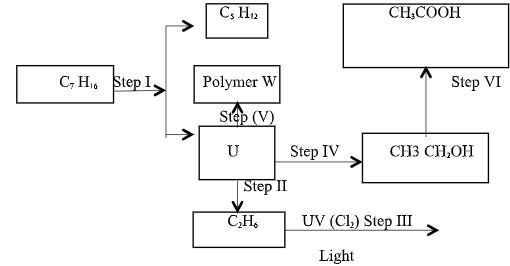
- Study the reaction scheme below and answer the questions that follow.
- Name the process labeled Step I (½ mark)
- Identify substance U (½ mark)
- State the name of the fifth member of the homologous series to which U belongs. (1 mark)
- Explain how acidified potassium manganite (VII) can be used to distinguish U from C2 H6. (2 marks)
- State one industrial application of the process in step II. (1 mark)
- Write the equation for the reaction in step III. (1 mark)
- Identify the reagent and condition required in step (IV) (1 mark)
- State one use of the polymer W. (1 mark)
represent two types of cleaning agents
- Name the class of the cleaning agent to which A belongs. (1 mark)
- Which cleaning agent would be suitable to use with water containing Magnesium Chloride? Explain (2 marks)
- Give the systematic names of the compounds whose structural formulae are given below.
-
- In an experiment 50cm3 of 1M copper (II) Sulphate solution was placed in a 100cm3 plastic beaker. The temperature of the solution was measured. Excess metal A powder was added to the solution, the mixture stirred and the maximum temperature recorded. The procedure was repeated using powders of metal B and C. The results obtained were given in the table below.
Metal A B C Maximum temperature(°C) 26.3 31.7 22.0 Initial temperature (°C) 22.0 22.0 22.0 - Arrange the metal A, B, C and Copper in ascending order of reactivity. (1mark) C, Copper, A B is the Ionic bond
- State one observation that was made when the most reactive metal than copper was added to the copper (II) Sulphate solution. (1mk)
- Other than temperature state two factors that affect rate of reaction 1mk
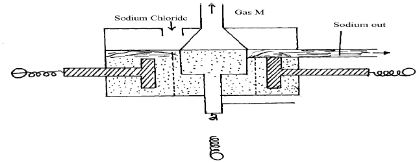
- The diagram below shows the extraction of sodium metal using the Down’s cell. Study it and answer the questions that follow.
- Explain why in this process sodium chloride is mixed with calcium chloride. (1 marks)
- Why is the anode made of graphite and not iron? (1 mark)
- State two properties of sodium metal that make it possible for it to be collected as shown in the diagram. (2 marks)
- What is the function of the steel gauze cylinder? (1 mark)
- Write ionic equations for the reactions which take place at
- Cathode (1 mark)
- Anode (1 mark)
- Why is sodium metal stored under kerosene? (1 mark)
- In an experiment 50cm3 of 1M copper (II) Sulphate solution was placed in a 100cm3 plastic beaker. The temperature of the solution was measured. Excess metal A powder was added to the solution, the mixture stirred and the maximum temperature recorded. The procedure was repeated using powders of metal B and C. The results obtained were given in the table below.
-
- Fractional distillation of liquid air is mainly used to obtain nitrogen and oxygen.
- Name one substance other than sodium hydroxide that is used to remove carbon (IV) oxide from the air before it is changed into liquid. (1 mark)
- Describe how nitrogen gas is obtained from the liquid air.
(Boiling points nitrogen = -196°C, Oxygen = -183°C ) (3 marks)
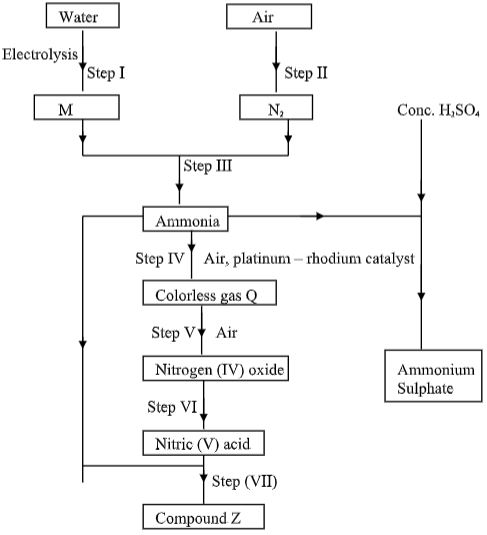
- Study the flow chart below and answer the questions that follow.
- Name substance M ……………………………(1 mark)
- Identify gas Q ……………………………………(1 mark)
- State one use of compound Z …………………(1 mark)
- A fertilizer manufacturing industry uses 1400dm3 of ammonia gas per hour to produce ammonium sulphate. Calculate the amount of ammonium sulphate produced in kg for one day if the factory operates for 18 hours.
( N = 14, H = 1, S = 32, O = 16, 1 mole of gas = 24dm3 ) (3 marks)
- Fractional distillation of liquid air is mainly used to obtain nitrogen and oxygen.
-
- Define the term Half -life ( 1mk)
- Table 2 contains information from the measurements made of the radioactivity in counts per minutes from a radioisotope iodine – 128.
Table 2Counts per min 240 204 180 156 138 122 108 Time (min) 0 5 10 15 20 25 40 - Plot a graph of counts per minute against time. (3marks)
- Use the graph to determine the half-life of iodine – 128. (1mark)
- What is the counts rate after 22 minutes? (½ mark)
- After how many minutes were the counts rate 160 counts per minute? (½ mark)
- Potassium has two isotopes
and radioactive
.
- State how the two isotopes differ. (1mark)
- The half-life of
is 1.3 x 109 years. Determine how long it would take for 4g of the isotope to decay to 1g. (1mark)
undergoes beta decay to form an isotope of calcium. Write the nuclear equation for this decay. (1mark)
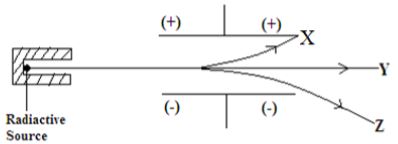
- Fig 2 shows how a radioactive material emitted radiations from its source. Study it and answer the questions that follow as shown below.
- Identify the radiation that: (2 marks)
- Has no mass?
- Contains Helium particles?
- State two applications of radioactivity in medicine (2 mks)
- Identify the radiation that: (2 marks)
-
- State the Hess’s law (1 mark)
- Use the information below to answer the questions that follow.
C(s) + O2(g) → CO2 Δ H = −394KJmol-1
H2(g) + ½ O2(g) → H2O(l) ΔH = −286KJmol-1
C2H2(g) +5O2(g) → 2CO2 + H2O ΔH = −1300KJmol-1- Draw an energy cycle diagram that links the heat of formation of ethyne with its heat of combustion and the heats of combustion of carbon and hydrogen. (2marks)
- Calculate the standard ‘enthalpy of formation’ of ethyne. (2mark)
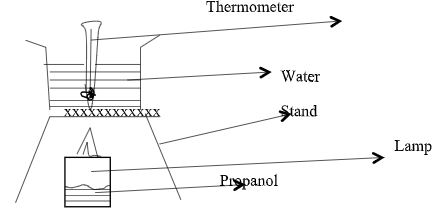
- The diagram below represents a set-up that was used in determining the molar heat of combustion of propanol. (C3H7OH) Thermometer
During the experiment the data given below was recorded.
Volume of water = 100cm3
Final temperature of water = 43.5°C
Initial temperature of water = 20.5°C
Mass of propanol + lamp before burning = 126.5g
Mass of propanol + lamp after burning = 124.7g
Calculate- The molar heat of combustion of propanol (3marks)
(Density of water = 1g/cm3, specific heat capacity of water = 4.2 kJ/kg/k, C=12.0, O = 16.0, H = 1.0) - The heating value of propanol. (1 mark)
- Give two disadvantages of using hydrogen as a source of fuel. (1 mark)
- The molar heat of combustion of propanol (3marks)
- Study the information given in the table below and answer the questions that follow.
Bond Bond energy in kJmol-1
C – H 414
Cl – Cl 244
C – Cl 326
H - Cl 431
Calculate the enthalpy change for the reaction. (2mks)
CH4(g)……+……4Cl2(g) → CCl4(g)……+……4HCl(g)
-
- Explain the meaning of the following in terms of oxidation numbers: (2mks)
- Reduction
- Oxidation
- Determine the oxidation number of chlorine in the ion. (1mk)
ClO3−
- The standard electrode potentials (Eø) of elements Dand G are-2.38Volts and −2.87 Volts respectively.
- Identify the strongest oxidizing agent 1mk
- Draw a labeled diagram of the cell formed when the two are connected. (3mks)
- Determine the e.m.f of the cell formed above. (2mks)
- During electrolysis of aqueous Copper(II) Sulphate using carbon electrodes a current of 2.0A was passed for 3 hours.
- Find the mass of copper metal deposited at the cathode (Cu=64;1F=96500) (3mks)
- State two factors that determine preferential discharge in electrolysis. (1 mark)
- Explain the meaning of the following in terms of oxidation numbers: (2mks)
MARKING SCHEME
-
- The grid below represents a periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements
- Write the formula of the compound formed by element J and M. (1mk)
- J2M/J2M2 accept Na2O/Na2O2
- Identify the least reactive element. Give a reason for your answer. (1mk)
- P-: Has stable electon arrangement of 2.8.8
- Compare the atomic size of K and O . Explain. (2mks)
- O -has smaller atomic size than k;o has more protons/stronger nuclear charge than k.OR
- K-has larger atomic size than O;K has fewer protons /weaker nuclear charge than O
- Write the formula of the compound formed by element J and M. (1mk)
- The following graph shows the reactivity of elements in period 3.
Explain the Trend at D and E(1mks)- Reactivity increases ;decrease in atomic radius/increase nuclear charge hence ease of gaining electrons
- The table below gives information on the Melting points of compounds of period 3 elements. The letters do not represent the actual symbols.
- Write the formula of
Elements R S T U V W Atomic number 11 12 13 14 15 16 M.pt of chloride °C 80` 714 − −70 −90 − 80 M.pt of oxide °C 1190 3080 2050 2750 560 −73 - Chloride of T (1mk)
- TCl3/T2Cl6 OR AlCl3/ Al3Cl6
- Oxide of U (1mk)
- UO2 OR SiO2
- Chloride of T (1mk)
-
- Using the information above,: suggest the type of bonding present in the chloride of V. Explain. (2mks)
- Covalent bonding; there is sharing of pair of electrons between V and chlorine
- The difference in melting point of chloride and oxide of U in terms of structure and bonding. (2mks)
- The chloride of U forms simple molecular structure; with weak Van der Waals forces that require less heat energy to break;
- The oxide of U forms giant atomic / covalent structure ;with strong covalent bond that require more heat energy to break.
- Why there is no melting point in the chloride of T. (1mk)
- It sublimes/ undergo sublimation
- Using the information above,: suggest the type of bonding present in the chloride of V. Explain. (2mks)
- Write the formula of
- The grid below represents a periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements
-
- Give the systematic names of the compounds whose structural formulae are given below.
-
- Butanoic acid (½ mark)
-
- Propan-1-ol (½ mark)
-
- Draw and name the structural formulae of the compound obtained when compounds in (a) react.
- Propylbutanoate (1 mark)
- Study the reaction scheme below and answer the questions that follow.
- Name the process labeled Step I (½ mark)
- Cracking
- Identify substance U (½ mark)
- Ethene
- State the name of the fifth member of the homologous series to which U belongs.(1 mark)
- Hexene/Hex-1-ene/Hex-2-ene/Hex-3-ene
- Explain how acidified potassium manganite (VII) can be used to distinguish U from C2H6. (2 marks)
- Add acidified potassium manganate VII to test tube containing substance U and bubble CH separately in a test tube; containing acidified potassium manganate VII;
- It changes from purple t colorless in test tube U; and it retains its purple color in CH
- State one industrial application of the process in step II. (1 mark)
- Manufacture of margarine
- Write the equation for the reaction in step III. (1 mark
- Identify the reagent and condition required in step (IV) (1 mark)
- Steam; catalyst phosphoric v acid, temperature 300c pressure 60-70 atm OR
- Concentrated sulphuric vi acid;water, warming
- State one use of the polymer (1 mark)
- Making of packing bags,plastics,insulating cables
- Name the process labeled Step I (½ mark)
represent two types of cleaning agents
- Name the class of the cleaning agent to which A belongs. (1 mark)
- Soapy detergents
- Which cleaning agent would be suitable to use with water containing Magnesium Chloride? Explain (2 marks
- B- Do not form scum in hard water/lathers easily with had water
- Name the class of the cleaning agent to which A belongs. (1 mark)
- Give the systematic names of the compounds whose structural formulae are given below.
-
- In an experiment 50cm3 of 1M copper (II) Sulphate solution was placed in a 100cm3 plastic beaker. The temperature of the solution was measured. Excess metal A powder was added to the solution, the mixture stirred and the maximum temperature was repeated using powder of metal B and C. The results obtained are given in the table below.
Metal A B C Maximum temperature(°C) 26.3 31.7 22.0 Initial temperature (°C) 22.0 22.0 22.0 - Arrange the metal A, B, C and Copper in ascending order of reactivity. (1mark)
- C, Copper, A B
- State one observation that was made when the most reactive metal than copper was added to the copper (II) Sulphate solution. (1mk) Ion
- Blue colour fades gradually to form colourless solution
- brown solid /residue deposited
- Other than temperature state two factors that affect rate of reaction 1mk
- concentration of reactants
- size of particle
- catalyst
- light
- Arrange the metal A, B, C and Copper in ascending order of reactivity. (1mark)
- The diagram below shows the extraction of sodium metal using the Down’s cell. Study it and answer the questions that follow.
- Explain why in this process sodium chloride is mixed with calcium chloride. (1 marks)
- Calcium chloride is an impurity it lowers mpt of sodium chloride from 80°C to 60°C
- Why is the anode made of graphite and not iron? (1 mark)
- It does not react with electrolyte/inert
- State two properties of sodium metal that make it possible for it to be collected as shown in the diagram. (2 marks)
- Less dense than molten sodium chloride;
- Low melting point than calcium so calcium crystalises out before sodium metal.
- What is the function of the steel gauze cylinder? (1 mark)
- Prevents sodium (from cathode)and chlorine(from anode)from recombining
- Write ionic equations for the reactions which take place at;
- Cathode (1 mark)
- 2Na + 2e → 2Na.
- Anode (1 mark)
- 2Cl → Cl2+ 2e
- Cathode (1 mark)
- Why is sodium metal stored under kerosene? (1 mark)
- Reacts explosively/spontaneously with air
- Explain why in this process sodium chloride is mixed with calcium chloride. (1 marks)
- In an experiment 50cm3 of 1M copper (II) Sulphate solution was placed in a 100cm3 plastic beaker. The temperature of the solution was measured. Excess metal A powder was added to the solution, the mixture stirred and the maximum temperature was repeated using powder of metal B and C. The results obtained are given in the table below.
-
- Fractional distillation of liquid air is mainly used to obtain nitrogen and oxygen.
- Name one substance other than sodium hydroxide that is used to remove carbon (IV) oxide from the air before it is changed into liquid. (1 mark)
- Potassium hydroxide
- Describe how nitrogen gas is obtained from the liquid air.
(Boiling points nitrogen = −196°C, Oxygen = −183°C ) (3 marks)- Heat/boil/warm the liquid air /rise the temperatures;
- Nitrogen comes out first ;because of its lower boiling point than oxy gen;
- Name one substance other than sodium hydroxide that is used to remove carbon (IV) oxide from the air before it is changed into liquid. (1 mark)
- Study the flow chart below and answer the questions that follow.
- Name substance M
- Hydrogen/H2 (1 mark)
- Identify gas Q
- Nitrogen ii oxide (rejec nitrogen monoxide) (1 mark)
- State one use of compound Z (1 mark)
- Fertilizer reject manufacture fertilizer
- A fertilizer manufacturing industry uses 1400dm3 of ammonia gas per hour to produce ammonium sulphate. Calculate the amount of ammonium sulphate produced in kg for one day if the factory operates for 18 hours.
( N = 14, H = 1, S = 32, O = 16, 1 mole of gas = 24dm3 ) (3 marks)
- Name substance M
- Fractional distillation of liquid air is mainly used to obtain nitrogen and oxygen.
- '
- Define the term Half -life 1mk
- Time taken for the mass /number of nucleotide to decay /reduce to half of its original mass/number
- Table 2 contains information from the measurements made of the radioactivity in counts per minutes from a radioisotope iodine – 128.
Table 2Counts per min 240 204 180 156 138 122 108 Time (min) 0 5 10 15 20 25 40 - Plot a graph of counts per minute against time. (3marks)
- Use the graph to determine the half-life of iodine – 128. (1mark)
- 27.5 minutes
- What is the counts rate after 22 minutes? (1/2 mark)
- 133 counts per minute
- After how many minutes were the counts rate 160 counts per minute? (1/2 mark)
- 13.5 minutes
- Potassium has two isotopes
and radioactive
.
- State how the two isotopes differ. (1mark)
- different number of neutrons ie 20 and 21 respectively
- The half-life of is 1.3 x 109years. Determine how long it would take for 4g of the isotope to decay to 1g. (1mark)
4g ----------------- 2g -----------------1g
1.3 × 10 9 × 2 = 2.6 × 109 years - undergoes beta decay to form an isotope of calcium. Write the nuclear equation for this decay.(1mark)
39 39 0
K Ca + e + energy
19 20 −1
- State how the two isotopes differ. (1mark)
- Fig 2 shows how a radioactive material emitted radiations from its source study it and answer the questions that follow as shown below.
- Identify the radiation that: (2MK)
- Has no mass - Y
- Contains Helium particles? Z
- State two applications of radioactivity in medicine 2mks
- Monitoring growth in bones and healing of fracture
- Destroy cancerous cells
- Sterilize surgicalequipment
- Provide power in heart pace setters
- Identify the radiation that: (2MK)
- Define the term Half -life 1mk
-
- State the Hess’s law (1 mark)
- Energy changes in converting reactants to products is the same regardless of the route in which a chemical change occurs.
- Use the information below to answer the questions that follow.
C(s) + O2(g) → CO2 Δ H = −394KJmol-1
H2(g) + ½ O2(g) → H2O(l) ΔH = −286KJmol-1
C2H2(g) +5O2(g) → 2CO2 + H2O ΔH = −1300KJmol-1- Write the equation for the formation of ethyne. (1 mark
- 2C + H2 → C2H2
- Draw an energy cycle diagram that links the heat of formation of ethyne with its heat of combustion and the heats of combustion of carbon and hydrogen. (3 marks)
- 2C + H2 → C2H2
- CO2 + H2O
Calculate the standard ‘enthalpy of formation’ of ethyne. (1 mark)
= + 226Kj/mole
- Write the equation for the formation of ethyne. (1 mark
- The diagram below represents a set-up that was used in determining the molar heat of combustion of propanol. (C3H7OH) Thermometer
During the experiment the data given below was recorded.
Volume of water = 100cm3
Final temperature of water = 43.5°C
Initial temperature of water = 20.5°C
Mass of propanol + lamp before burning = 126.5g
Mass of propanol + lamp after burning = 124.7g
Calculate- The molar heat of combustion of propanol (3 marks)
(Density of water = 1g/cm3, specific heat capacity of water = 4.2 Kj/kg/k, C=12.0, O = 16.0, H = 1.0)
H = Mc ΔT
0.1 × 4.2 × 23.0 = 9.66 Kj
Moles = 1.8 ÷ 60 = 0.03 moles
9.66 ÷ 0.03 = -322 kJ/mole - The heating value of propanol. (1 mark)
322 = 5.3Kj/g
60 - Give two disadvantages of using hydrogen as a source of fuel. (1 mark
- Expensive as it involves electrolysis
- Storage complication because its lighter
- Not safe highly flammable/volatile/no smell to detect leakage
- Not easy to transport
- Depends on fossil fuel
- The molar heat of combustion of propanol (3 marks)
- Study the information given in the table below and answer the questions that follow.
Bond Bond energy in kJmol-1
C – H 414
Cl – Cl 244
C – Cl 326
H - Cl 431
Calculate the enthalpy change for the reaction. (2mks)
ΔH = (1 × bond energy (C-H) + 1 × bond energy (Cl-Cl)) - (1 × bond energy (C-Cl))
ΔH = (1 × 414 kJ/mol + 1 × 244 kJ/mol) - (1 × 326 kJ/mol)
ΔH = (414 kJ/mol + 244 kJ/mol) - 326 kJ/mol
ΔH = 658 kJ/mol - 326 kJ/mol
ΔH = 332 kJ/mol
So, the enthalpy change for the reaction CH is 332 kJ/mol.
- State the Hess’s law (1 mark)
-
- Explain the meaningofthefollowing in terms of oxidation numbers: (2mks)
- Reduction
- Decrease in oxidation number.
- Oxidation
- Increase in oxidation number
- Determinethe oxidation number of chlorine in the ion. (1mk)
Cl + 3× −2 = −1
= +5
- Reduction
- The standard electrode potentials(Eø) of elements D and G are −2.38Volts and−2.87volts respectively
- Identify the strongest oxidizing agent
- Element G with an E° of −2.87 V is the stronger oxidizing agent.
- Draw a labeled diagram of the cell formed when the two are connected. (3mks)
|------------------- Anode (oxidation) - Element D (-2.38 V)
| Electrons (e^-)
| Salt bridge (or ion-conductive bridge)
| Electrons (e^-)
|------------------- Cathode (reduction) - Element G (-2.87 V) - Determine the e.m.f of the cell formed above.(2mks)
Ecell = Ered − Eoxd
−2.38 − − 2.87 = +0.49 v
- Identify the strongest oxidizing agent
- During electrolysis of aqueous copper(II)Sulphate using carbon electrodes a current of 2.0A was passed for 3hours.
- Find the mass of copper metal deposited at the cathode(Cu=64;1F=96500) (3mks)
Q = It
2.0 ×.3 ×3600 = 21600 c 21600× 64
Cu2 + + 2e cu 19300
2 × 96500 = 193000c = 7.16g - State two factors that determine preferential discharge in electrolysis.
- nature of electrodes
- Concentration of ions
- Position of the ion in electrochemical series (1mk)
- Find the mass of copper metal deposited at the cathode(Cu=64;1F=96500) (3mks)
- Explain the meaningofthefollowing in terms of oxidation numbers: (2mks)
Download Chemistry Paper 2 Questions and Answers - Samia Joint Mock Examination 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students