Instructions to candidates
- Answer ALL the questions
- KNEC Mathematical tables and electronic calculators may be used for calculations.
- All working MUST be clearly shown where necessary.
- Candidates should answer the questions in English.
- What is the difference between chromatography and chromatology? (1mark)
- When dilute Sulphuric (VI) acid is connected in a circuit to test conduction of electricity, the bulb lights while when concentrated Sulphuric (VI) acid is used in the same set-up, the bulb does not light. Explain this observation. (2marks)
- Explain why Aluminium Chloride has PH 3 when dissolved in water? (2marks)
- Below is a list of substances.
Soap solution, common salt, urine, lemon juice and baking powder.
Select:- A substance that is likely to give a PH of 3.0 when tested? (1mark)
- A substance (s) which is likely to resemble sodium hydrogen carbonate.(1mark)
- Two substances when reacted are likely to give the product with same PH as that of common salt. (1mark)
- Briefly explain the observation made when a small piece of sodium metal is dropped into a bowl of water. (3marks)
-
- Define Le Chatelier’s principle. (1mark)
- A fixed mass of a gas has a volume of 400cm2 at 20°C, what temperature rise would produce a 10% increase in volume if the pressure remains constant. (3marks)
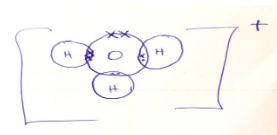
- Using Dots (.) and (x) diagram, show the number of electrons used in bonding of H3O+ (2marks)
- Explain why a luminous flame appears yellow. (2marks)
- Some sodium chloride was found to be contaminated with copper (II) oxide. Describe how a dry sample of sodium chloride can be separated from the mixture. (2marks)
- Hot platinum wire was lowered into a flask containing concentrated ammonia solutions shown below.
State and explain observations made (3marks) - Give three characteristics of gases according to Kinetic theory of matter. (3marks)
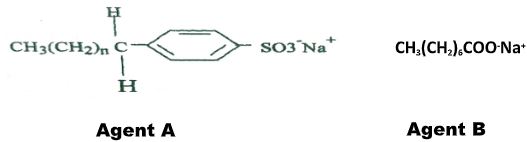
- The formula below represents active ingredients of two cleansing agents A and B
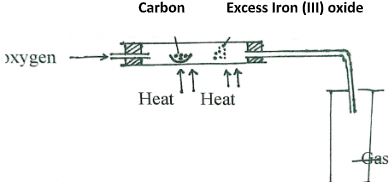
Which one of the cleansing agents would be suitable to be used in water containing magnesium hydrogen carbonate? Explain. (2marks) - The set-up below was used to obtain a sample of iron
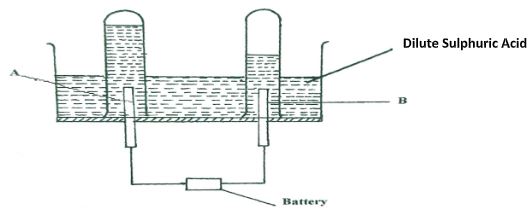
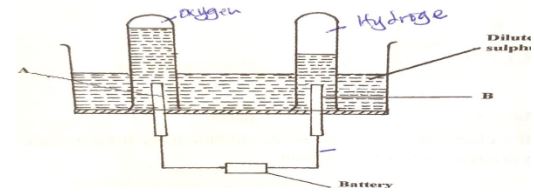
Write two equations for the reactions which occur in the combustion tube. (2marks) - The diagram below represents a set-up that can be used for the electrolysis of dilute Sulphuric acid.
- Name the electrodes A and B (1mark)
- Write an equation for the reaction taking place at electrode B. (1mark)
- What happens to the concentration of dilute sulphuric acid as the reaction continues? (1mark)
- Describe one physical and one chemical test that can be used to identify Ethane gas. (2marks)
- 15cm3 of a solution containing 2.88g/dm3 of an alkali XOH completely reacts with 20cm3 of 0.045M sulphuric acid. Calculate the reactive atomic mass of X present in the alkali. (3marks)
- Using equations, state and explain the changes in mass that occur when the following are heated separately in open crucible. (3marks)
- Magnesium metal
- Zinc carbonate
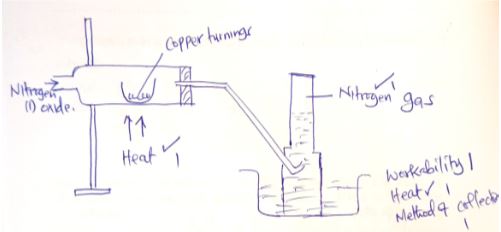
- In the space provided below, draw a set-up that can be used to show the reaction between nitrogen (I) oxide with copper to give Nitrogen gas. (3marks)
- The flow chart below shows some process in extraction of lead metal. Study it and answer the questions that follow;
- Name two raw materials that were fed into Unit I (1mark)
- State one environment hazard associated with the process in Unit I. (1mark)
- What is the function of Coke in Unit II (1mark)
- Sulphur exhibits as an allotropy.
- What is allotropy? (1mark)
- Name the two allotropes of sulphur. (1mark)
- Sulphur powder was placed in a deflagrating spoon and heated on a Bunsen burner.
- State the observation made. (1mark)
- The product obtained was dissolved in water. Comment on the PH of the solution formed. (1mark)
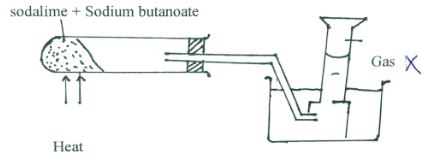
- The set-up below was used to prepare a sample of an organic compound X.
- Identify gas X (1mark)
- Write the equation for the reaction that produces gas X. (1mark)
- 1 Mole of chlorine was reacted with gas X in presence of sunlight.
- State one observation made. (½ mark)
- Name the major product formed. (½ mark)
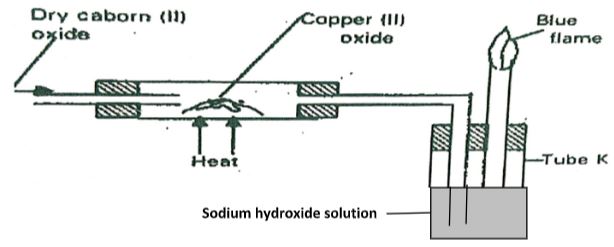
- The apparatus shown below was used to investigate the effect of carbon (II) oxide on Copper (II) oxide.
- State the observation that was made in the combustion tube at the end of the experiment. (1mark)
- Write an equation for the reaction that took place in the combustion tube. (1mark)
- Why is it necessary to burn the gas coming out of tube K? (1mark)
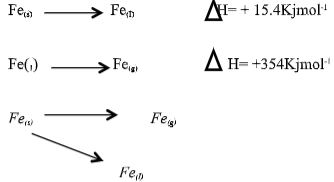
- The equation below represents changes in the physical state of ions metal:
Fe(s) → Fe(I) ΔH = + 15.4Kjmol-1
Fe(1) → Fe(g) ΔH = + 354Kjmol-1
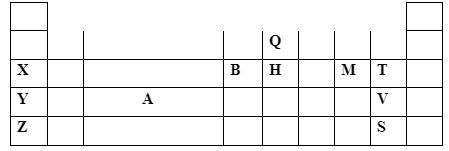
Calculate the amount of heat energy required to change 10kg of solid iron to gaseous iron. (Fe = 56) (3marks) - The section below represents part of the periodic table. Study it and answer the questions that follow; the letters are not the actual symbol of the elements.
- Explain why the atomic radius of T is smaller than that of M (1mark)
- Compare the electrical conductivity of element X and B. (2marks)
- Read the following passage and answer the questions.
A salt X was heated with slaked lime (calcium hydroxide). A colorless gas R with a characteristic smell that turns red litmus paper blue was evolved. A large quantity of this gas was passed through an inverted filter funnel into Copper (II) sulphate solution, and a deep blue solution M was obtained.- Identify gas R (1mark)
- What is X most likely to be? (1mark)
- Write an equation for the reaction between X and slaked lime. (1mark)
- Consider the following reaction:
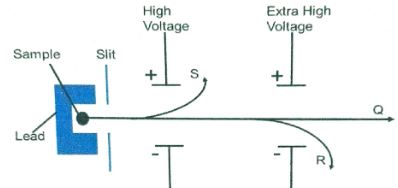
Sketch an energy level diagram showing the relative activation energies for the catalyzed and uncatalyzed reactions using the axes below. (2marks) - The diagram below shows the radiations emitted by a radioactive sample.
- Identify radiation particles S and R (2marks)
-
- Starting with red roses, describe how a solution containing the red pigments may be prepared? (2marks)
- How can the solution be used as an indicator? (1mark)
-
- Give one reason why some of the laboratory apparatus are made of ceramics. (1mark)
- Name the two apparatus that can be used to measure approximately 75cm3 of dilute sulphuric (VI acid. (2marks)
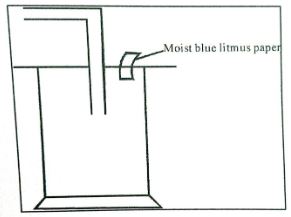
- Dry chlorine was collected using the set-up below.
- Name a suitable drying agent for chlorine gas? (1mark)
- State one property of chlorine gas which facilitates this method of collection. (1mark)
- State one observation made on the moist blue litmus paper. (1mark)
MARKING SCHEME
- What is the difference between chromatography and chromatology? (1mark)
- Chromatography is the method of separating coloured substance while chromatology is the study of colours.
- When dilute Sulphuric (VI) acd is connected in a circuit to test conduction of electricity, the bulb lights while when concentrated Sulphuric (VI) acid is used in the same set-up, the bulb does not light. Explain this observation. (2marks)
- Coonc. Sulphuric (vi)acid is molecular hence ions are fixed while Dil. H2SO4 dissociatesin water providing H+ and SO42-that conducts electricity.
- Explain why Aluminium Chloride has PH 3 when dissolved in water? (2marks)
- AlCl3 hydrolysis in water to form Al2Cl6 which is acidic.
- Below is a list of substances.
Soap solution, common salt, urine, lemon juice and baking powder.
Select:- A substance that is likely to give a PH of 3.0 when tested? (1mark)
- Lemon juice
- A substance (s) which is likely to resemble sodium hydrogen carbonate.(1mark)
- Baking powder.
- Two substances when reacted are likely to give the product with same PH with common salt. (1mark)
- Baking powder and lemon juice
(accept – Soap solution and lemon)
- Baking powder and lemon juice
- A substance that is likely to give a PH of 3.0 when tested? (1mark)
- Briefly explain the observation made when a small piece of sodium metal is dropped into a bowl of water. (3marks)
- It floats and darts on surface of water
- T moves and melts a silvery ball.
- It produces a hissing sound
-
- Define Le Chatelier’s principle. (1mark)
- When a change in condition is applied to a system in equilibrium, the system moves so as to oppose that change.
- A fixed mass of a gas has a volume of 400cm2 at 20°C, what temperature rise would produce a 10% increase in volume if the pressure remains constant. (3marks)
V1 = V2
T1 T2 Conversion of volume by 10% = 1
400 = 440
293 T2
T2 = 440 x 293 = 3223/49.3°C
400
- Define Le Chatelier’s principle. (1mark)
- Using Dots (.) and (x) diagram, show the number of electrons used in bonding of H3O+ (2marks)
- Explain why a luminous flame appears yellow. (2marks)
- Because of unburnt carbon particle which glows yellow when burnt.
- Some sodium chloride was found to be contaminated with copper (II) oxide. Describe how a sample of sodium chloride can be separated from the mixture. (2marks)
- Add water to the mixture to dissolve Nacl.
- Filter to obtain Nacl as filtrate and CuO as residue.
- Evaporate the filtrate in evaporating dish over a water bath to obtain dry sample.
- Hot platinum wire was lowered into a flask containing concentrated ammonia solutions shown below.
State and explain observations made (3marks)- Hot platinum wire continues to glow red.
- Brown fumes are observed
- Hot platinum glows red since reaction of oxygen and NH3is exothermic.
- Brown fumes since NH3 is oxidized in presence of Pt catalyst to produce NO which is further oxidized to NO2
- Give three characteristics of gases according to Kinetic theory of matter. (3marks)
- Continuous random motion
- Widely spread in space.
- Weak forces of attraction between molecules.
- The formula below represents active ingredients of two cleansing agents A and B
Which one of the cleansing agents would be suitable to be used in water containing magnesium hydrogen carbonate? Explain. (2marks)- Agent A
- The set-up below was used to obtain a sample of iron
Write two equations for the reactions which occur in the combustion tube. (2marks)- 2C + O2 → CO2
- 2CO + Fe2 O3 → 2 Fe + CO2
- The diagram below represents a set-up that can be used for the electrolysis of dilute Sulphuric acid.
- Name the electrodes A and B (1mark)
- A – Anode.
- B – Cathode
- Write an equation for the reaction taking place at electrode B. (1mark)
- 4H+(aq) + 4e(aq) → 2H2(g) / 2H+(aq) + 2e → H2(g)
- What happens to the concentration of dilute sulphuric acid as the reaction continues? (1mark)
- The concentration increases.
- Name the electrodes A and B (1mark)
- Describe one physical and one chemical test that can be used to identify ethane gas. (2marks)
- Physical - Burns with a yellow sooty flame.
- Chemical - Decolorizes purple acidified KMnO4/
- Decolorizes yellow bromine water
- 15cm3 of a solution containing 2.88g/dm3 of an alkali XOH completely reacts with 20cm3 of 0.045M sulphuric acid. Calculate the reactive atomic mass of X present in the alkali. (3marks)
2XOH + H2SO4 → X2?SO4 + H2O
15 20
Moles of H2SO4 = 20 x 0.045 = 0.0009 moles
1000
Mols of XOH = 0.0009 x 2 = 0.0018
Molarity of XOH = M x 1000 = (0.0018 x 1000)
V 15
= 0.12M
RFM = Concing/dmo
Conc in m
= 2.88
0.12
= 24
X + 16 + 1 = 24
X = 7 - Using equations state and explain the changes in mass that occur when the following are heated separately in open crucible. (3marks)
- Magnesium metal
- In magnesium the mass increases
- @Mg(s) + O2(g) 2MgO(g)/ 3Mg(s) + N2 Mg3N2(s)
- Zinc carbonate
- Zinc carbonate mass decreases.
ΔH - ZnCO3(s) → ZnO(s) + CO2(g)
- Zinc carbonate mass decreases.
- Magnesium metal
- In the space provided below, draw a set-up that can be used to show the reaction between nitrogen (I) oxide with copper to give Nitrogen gas. (3marks)
- The flow chart below shows some process in extraction of lead metal. Study it and answer the questions that follow;
- Name two raw materials that were fed into Unit I (1mark)
- Lead Sulphide
- Air/Oxygen
- State one environment hazard associated with the process in Unit I. (1mark)
- SO2 leads to formation of acidic rain
- SO2 Causes respiratory infection
- What is the function of Coke in Unit II (1mark)
- Reduces lead (II) oxide into lead metal
- Name two raw materials that were fed into Unit I (1mark)
- Sulphur exhibits as an allotropy.
- What is allotropy? (1mark)
- Existence of element in more than one structural form in the same physical state.
- Name the two allotropes of sulphur. (1mark)
- Rhombic
- Monoclinic
- Sulphur powder was placed in a deflagrating spoon and heated on a Bunsen burner.
- State the observation made. (1mark)
- Blue flame/pungent smell
- The product obtained was dissolved in water. Comment on the PH of the solution formed. (1mark)
- Acidic/low PH
- State the observation made. (1mark)
- What is allotropy? (1mark)
- The set-up below was used to prepare a sample of an organic compound X.
- Identify gas X (1mark)
- Propane
- Write the equation for the reaction that produces gas X. (1mark)
- CH3Ch2CH2COONa + NaOH → Na2CO3 + C3H8 (ignore state symbols)
- 1 Mole of chlorine was reacted with gas X in presence of sunlight.
- State one observation made. (½ mark)
- Green – Yellow chlorine turns Yellow
- Name the major product formed. (½ mark)
- Chloropropane
- State one observation made. (½ mark)
- Identify gas X (1mark)
- The apparatus shown below was used to investigate the effect of carbon (II) oxide on Copper (I) oxide.
- State the observation that was made in the combustion tube at the end of the experiment. (1mark)
- Black solid turns brown
- Write an equation for the reaction that took place in the combustion tube. (1mark)
- CO(g) + CuO(s) → Cu(g) + CO2(g)
- Why is it necessary to burn the gas coming out of tube K? (1mark)
- To avoid pollution when exposed to air.
- State the observation that was made in the combustion tube at the end of the experiment. (1mark)
- The equation below represents changes in the physical state of ions metal:
Calculate the amount of heat energy required to change 10kg of solid iron to gaseous iron. Fe = 56 (3marks)
I mole of Fe(56) required 154 + 354 = 396.5KJ/
10,000g =?
10,000 x 360.4 = 65964.285KJ.
56 - The section below represents part of the periodic table. Study it and answer the questions that follow; the letters are not the actual symbol of the elements.
- Explain why the atomic radius of T is smaller than that of M (1mark)
- T has more protons hence the nuclear charge than M attracting outermost electrons closer to the nucleus reducing atomic radius.
- Compare the electrical conductivity of element X and B. (2marks)
- B has higher conductivity than X since B has three/more delocalized electrons than X which has one/less.
- Explain why the atomic radius of T is smaller than that of M (1mark)
- Read the following passage and answer the questions.
A salt X was heated with slaked lime (calcium hydroxide). A colorless gas R with a characteristic smell that turns red litmus paper blue was evolved. A large quantity of this gas was passed through an inverted filter funnel into Copper (II) sulphate solution, and a deep blue solution M was obtained.- Identify gas R (1mark)
- Ammonia gas
- What is X most likely to be? (1mark)
- Ammonium chloride.
- Write an equation for the reaction between X and slaked lime. (1mark)
- Ca(OH)2(aq) + NH4Cl(aq) CaCl2(aq) + 2NH3(g) + H2O(I)
- Identify gas R (1mark)
- Consider the following reaction:
Sketch an energy level diagram showing the relative activation energies for the catalyzed and unanalyzed reactions using the axes below. (2marks) - The diagram below shows the radiations emitted by a radioactive sample.
Identify radiation particles S and R (2marks)- S Alpha accept symbols
- R Beta
-
- Starting with red roses, describe how a solution containing the red pigments may be prepared? (2marks)
- Crush roses using mortar and pestle to obtain smooth paste
- Add propanone /ethanol and continue crushing to dissolve red pigment
- Filter to obtain red pigment solution
- Place under the sun for propanone to evaporate.
- How can the solution be used as an indicator. (1mark)
- Add drops of pigments in different sample of acid and bases ½ hence show some colour in acid but different colour in acid ½
- Starting with red roses, describe how a solution containing the red pigments may be prepared? (2marks)
-
- Give one reason why some of the laboratory apparatus are made of ceramics. (1mark)
- They do not react with acids and bases.
- Name the two apparatus that can be used to measure approximately 75cm3 of dilute sulphuric (VI acid. (2marks)
- Pipette
- Burette
- Measuring cylinder
- Give one reason why some of the laboratory apparatus are made of ceramics. (1mark)
- Dry chlorine was collected using the set-up below.
- Name a suitable drying agent for chlorine gas? (1mark)
- Conc. Sulphuric (iv) acid/anhydrous calcium chloride.
- State one property of chlorine gas which facilitates this method of collection. (1mark)
- Slightly denser than air.
- State one observation made on the moist blue litmus paper. (1mark)
- It turns red then decolourises
- Name a suitable drying agent for chlorine gas? (1mark)
Download Chemistry Paper 1 Questions and Answers - Samia Joint Mock Examination 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students