INSTRUCTIONS TO CANDIDATES.
- Answer ALL the questions in the spaces provided.
- You are not allowed to start working with the apparatus for the first 15 minutes of the 2¼ hours allowed time for the paper.
- Use the 15 minutes to read through the question paper and make sure that you have all the chemicals and apparatus that you may require.
- Mathematical tables and electronic calculators may be used.
- All working MUST be clearly shown where necessary.
Question 1
You are provided with:-
- 2.0 g solid A.
- 2.0 M hydrochloric acid solution B.
- 0.1M Sodium hydroxide solution
You are required to determine the;- Enthalpy change (∆H )for the reaction between solid A and one mole of hydrochloric acid.
PROCEDURE I
Using a burette,place 20.0cm3 of 2.0M hydrochloric acid,solution B in a 100cm3 plastic beaker.Measure the temperature of the solution after every half-minute and record the values in table 1.At exactly 2 ½ minutes, add all of solid A to the acid and stir the mixture gently with the thermometer. Measure the temperature of the mixture after every half-minute and record the values in table 1.Retain the mixture for use in procedure II.
Table 1
| Time (mins) | 0 | ½ | 1 | 1½ | 2 | 2½ | 3 | 3½ | 4 | 4½ |
| Temperature °C |
(3mks)
- Plot a graph of temperature against time. (3mks)
Using the graph, determine the change in temperature (∆T) (1mk) - Calculate heat change for the reaction.(Assume specific heat capacity of the mixture is 4.2J/g/K, Density of solution=1.0g/cm3)) (2mks)
Procedure II
Rinse the burette thoroughly and fill with sodium hydroxide solution. Transfer all the contents of 100cm3 plastic beaker used in procedure I into a 250cm3 volumetric flask. Add distilled water to make up to the mark. Label this solution C.
Using a pipette and pipette filler, place 25cm3 of solution C into a clean conical flask and add 2 drops of phenolphthalein indicator and titrate against sodium hydroxide solution. Record your results in table 2 below. Repeat the titration two more times to complete the table 2 below.
TABLE I
| 1 | 2 | 3 | |
| Final burette reading (cm3) | |||
| Initial burette reading (cm3) | |||
| Volume of sodium hydroxide solution used (cm3) |
(4mks)
- Determine the average volume of sodium hydroxide solution A used. (1 mk)
- Calculate number of moles of;
- sodium hydroxide used (1 mk)
- hydrochloric acid in 25cm3 of solution C . (1 mk)
- hydrochloric acid in 250cm3 of solution C . (1 mk)
- hydrochloric acid in 20cm3 of solution B. (1 mk)
- hydrochloric acid that reacted with solid A. (1 mk)
- Calculate the enthalpy of reaction between solid A and one mole of hydrochloric acid solution B. (1mk)
Question 2
You are provided with solid E,F and G. Carry out the tests below and write the observations and inferences in the spaces provided.
- Place all solid E in a boiling tube and add about 15cm3 of distilled water. Shake the boiling tube until all the solid dissolves. Label this solution E. Divide the solution E into 4 portions.
Observations Inferences (1mk) (1mk) - To the first portion of solution E in a test tube, add 4 drops of 2M sulphuric (VI) acid.
Observations Inferences (1mk) (1mk) - To the second portion of solution E in a test tube,add sodium hydroxide drop wise until in excess.
Observations Inferences (1mk) (1mk)
- To the first portion of solution E in a test tube, add 4 drops of 2M sulphuric (VI) acid.
- Place one half of solid F in a test tube .Add 2cm3 of distilled water and shake well. Add 3 drops of this solution to the third portion of solution E.
Observations Inferences (1mk) (1mk) - To the fourth portion of solution E in a test tube ,add 2 drops of aqueous potassium iodide.
Observations Inferences (1mk) (1mk) - Name the cation present in solid E……………………………….. (1mk)
Question 3
You are provided with solid G. Carry out the tests below and write the observations and inferences in the spaces provided.
- Using a metallic spatula, place a third of solid G and ignite on a non-luminous flame.
Observations Inferences (1mk) (1mk) - Place the remaining solid G in a boiling tube. Add 10cm3 of distilled water and shake well. Label this solution G. Use the solution G for the tests below.
- To the first portion of solution G in a test tube, determine its pH value.
Observations Inferences (1mk) (1mk) - To the second portion of solution G in a test tube,addd 3 drops of acidified potassium manganite (VII).
Observations Inferences (1mk) (1mk) - To the third portion of solution G in a test tube, add 2 drops of bromine water.
Observations Inferences (1mk) (1mk) - To the fourth portion of solution G in a test tube,add the remaining solid F.
Observations Inferences (1mk) (1mk)
- To the first portion of solution G in a test tube, determine its pH value.
CONFIDENTIAL
- 2.0 g solid A weighed accurately and supplied in a stoppered container.
- About 60cm3 solution B
- About 130cm3 Sodium hydroxide solution
- One thermometer -100C - 1100C
- One Stop watch
- One 100ml plastic beaker
- One burette 0-50ml
- One pipette
- One volumetric flask
- About 500ml distilled water
- 3 labels
- 2 Conical flasks
- One 10ml measurimg cylinder
- One 100ml measurimg cylinder
- One boiling tube
- 0.5g Solid E
- 6-clean dry test tubes
- 0.2g Solid F
- 0.5g Solid G
- pH Chart
- Metallic spatula
- Six droppers
ACCESS TO
- 2M NaOH
- 2M Sulphuric (VI) acid
- 0.5M Potassium iodide
- Bromine water
- Barium chloride soln. (BaCl2)
- Universal indicator soln
- Acidified KmnO4.
- Phenolphthalein indicator
- Source of heating
NOTES
- Solid A —Borax salt.
- Solid E-Lead Nitrate
- Solid F-Sodium Carbonate
- Solid G-.Maleic acid
PREPARETIONS
- Solution B is prepared by adding 172cm3 (1.18g/cm3)of concentrated hydrochloric acid to about 500ml distilled water and diluting to 1 Litre solution.
- Sodium hydroxide solution is prepared by dissolving 4gms of the soild in about 500ml of water then diluting to 1 Litre (0.1 M).
- Bromine water is prepared 1ml of liquid water and dissolving it 100ml of distilled water in a fume chamber.(Freshly Prepared).
- Acdified KMnO4 is prepared by dissolving 3.16g of solid potassium manganate(VII) in about 200ml of 2M Sulphuric (VI) acid and top up to 1 Litre of distilled water.
MARKING SCHEME
Question 1
PROCEDURE 1
Table 1
| Time (mins) | 0 | ½ | 1 | 1½ | 2 | 2½ | 3 | 3½ | 4 | 4½ |
| Temperature °C | 20 | 20 | 20 | 20 | 20 | X | 16 | 17 | 18 | 19 |
CT = 1
TR = 1
D = 1
-
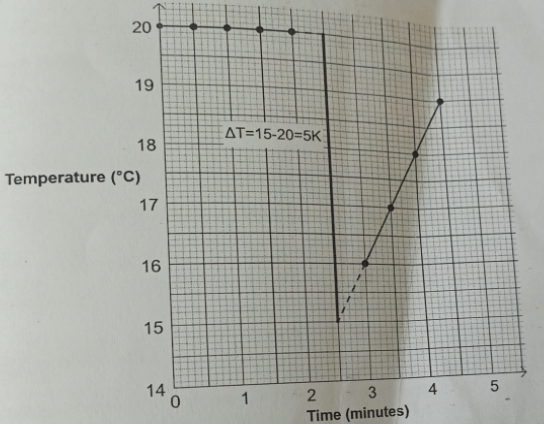
Change in temperature
20 − 10 = 5.0 (after extrapolation) - ΔH = 0.42kJ
PROCEDURE II
TABLE I
| 1 | 2 | 3 | |
| Final burette reading (cm3) | 15.1 | 30.1 | 45.0 |
| Initial burette reading (cm3) | 0.0 | 15.1 | 30.1 |
| Volume of sodium hydroxide solution used (cm3) | 15.1 | 15.0 | 14.9 |
CT = 1
D = 1
AC = 1
PA = 1
FA = 1
- 15.0cm3
-
- = 0.0015 moles
- Mole ratio 1:1 hence 0.0015 moles HCl solution C used
- = 0.015 moles
- = 0.04 moles
- 0.04 − 0.015 = 0.025 moles
- = + 16.8 kJ/mole
Question 2
-
Observations Inferences Solid E dissolves to form a colourless solution
(1mk)- A soluble salt present
- Cu2+,Fe2+,Fe3+ absent
(1mk)-
Observations Inferences A white precipitate formed
(1mk)Ca2+ ,Pb2+ ,Ba2+ present
(1mk) -
Observations Inferences A white precipitate dissolves in excess
(1mk)Pb2+ present
(1mk)
-
-
Observations Inferences A white precipitate formed
(1mk)Cl- ,CO32- ,SO42- ,SO32- present
(1mk) -
Observations Inferences A yellow precipitate
(1mk)Pb2+ present
(1mk)- Solid E = Lead (II) ions (1mk)
Question 3
-
Observations Inferences Solid G melts and burns with a yellow sooty flame
(1mk)
(1mk) -
-
Observations Inferences pH value is 1,2
(1mk)Solution is strongly acidic
(1mk) -
Observations Inferences Purple colour of potassium manganite(VII)
solution changes to colourless
(1mk)R -OH Present(1mk)
-
Observations Inferences Yellow colour of bromine water changes to colourless
(1mk)(1mk) -
Observations Inferences Effervescence occurs. (1mk) CO32- Present (Tied to part 2(b) above). (1mk)
-
Download Chemistry Paper 3 Questions and Answers - Momaliche Pre Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
