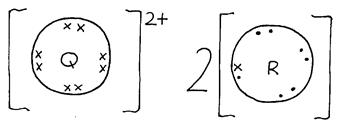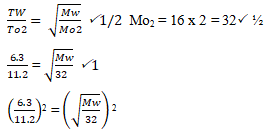INSTRUCTIONS TO CANDIDATES
- Answer all the questions.
- Mathematical table and electronic calculators may be used
- All workings must be clearly shown where necessary
- Answer all questions in English.
- The products formed by the action of heat on carbonates A,B, and C are shown below.
Carbonates Products Fromed A Metal Oxide + Carbon (IV) Oxide B Metal, Oxygen and Carbon (IV) Oxide C No product - Arrange the metals in order of reactivity starting with the most reactive. ( 2 mks)
- Which of the carbonate is soluble in water? (1 mk)
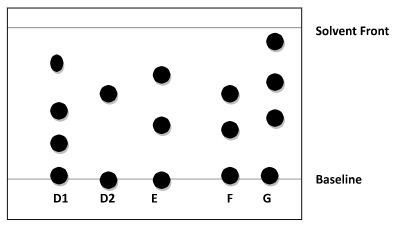
- Samples of urine from three participants E, F and G at a national police recruitment exercise were spotted onto a chromatography paper alongside two illegal drugs D1 and D2. A chromatogram was run using ethanol. The diagram below shows the chromatogram.
- Identify the participant who had used an illegal drug. (1 mk)
- Which drug is less soluble in ethanol? (1 mk)
- Describe a simple laboratory experiment that can be used to distinguish between Sodium sulphite and Sodium Sulphate. (3 mks)
- Explain why burning Magnesium ribbon continues to burn in a gas jar full of sulphur (iv) Oxide while a burning wooden splint would be extinguished. (3 mks)
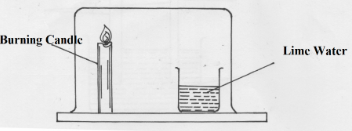
- Study the arrangement below and answer the questions that follow
State and explain what will be observed after sometime. (2 mks) - Study the diagram below and answer the questions that follow.
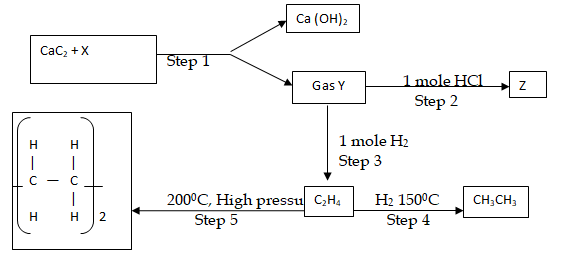
- Identify reagent X. (1 mk)
- Draw the structural formula of gas Y. (1 mk)
- What name is given to the process that takes place in step 5? (1 mk)
- Describe a laboratory experiment that can be used to obtain aluminum chloride from a mixture of sodium chloride and aluminum chloride. (2 mks)
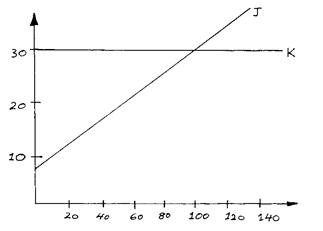
- The graph below shows the solubility curves for salts J and K.
Which of the two salts is more soluble in water? Explain (2 mks) - The electronic configuration of the ions of L2+ and M - are 2.8 and 2.9 respectively
- Write the electronic configuration of atoms of element L and M. (1 mk)
- Write the formula of the Oxide of L ( 1 mk)
- Compare the atomic radius of the element M and the ionic radius of ion M - (1 mk)
- Iron roofing sheets are coated with zinc as “Sacrificial” metal.
- Give the name of the process by which iron sheets are coated with zinc. (1 mk)
- Give a reason why copper is not used as “ sacrificial” metal in the process you have named in (a) above. (2 mks)
- The empirical formula of a hydrocarbon is C 2 H 3 . It has a molecular mass of 54.
- Determine the molecular formula of the hydrocarbon. (1 mk)
( C= 12, H= 1) - Draw the structural formula and name the hydrocarbon. (2 mks)
- Determine the molecular formula of the hydrocarbon. (1 mk)
- Write chemical equations to show the difference between the bleaching action by chlorine and bleaching action by sulphur (IV) Oxide gases. (2 mks)
- Elements Q and R have atomic numbers 12 and 17 respectively.
- Which element is a metal. (1 mk)
- Which dot(.) and (x)cross to represent electrons, show the bonding between Q and R in the compound of the elements. (2 mks)
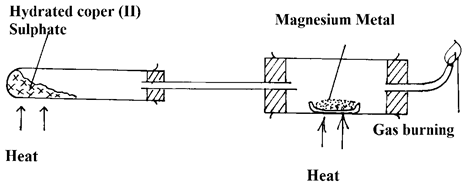
- Study the set- up below and answer the questions that follow:-
- What is the role of hydrated copper (II) Sulphate in the set- up. (1 mk)
- Identify Gas S? (1 mk)
- Write a chemical equation for the reaction taking place in the combustion tube. (1 mk)
- Calculate the number of aluminum ions in 250 cm 3 of 0.1 M aluminum sulphate.
( Avogadro’s Constant = 6.0 x1023 ) (3 mks) - The table below shows solutions and their PH values.
Solution pH Value T 1.5 U 7.0 V 14.0 - Select any pair that would react to form a solution of PH 7. (1 mk)
- Identify two solutions that would react with Alluminium hydroxide. Explain your answer. (2 mks)
- Name two allotropes of carbon. (2 mks)
- 20cm3 of gas W takes 12.6 seconds to pass through a orifice. 10cm3 of Oxygen gas takes 11.2 seconds to diffuse through the same orifice under same conditions of temperature and pressure calculate the molecular mass of gas W. (2 mks)
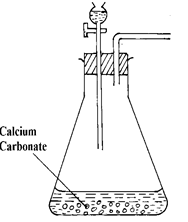
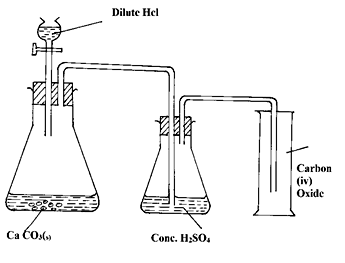
- The diagram below shows an incomplete set- up of the laboratory preparation of dry carbon (IV) Oxide. Complete the diagram. (3 mks)
- State three uses of Argon (3 mks)
- The table below shows the results obtained when halogens are bubbled into a test tube containing solutions of halides labeled A, B and C. A tick ( ✓ ) means reaction takes place and (x) no reaction occurs.
Halogen Halide ion in solution A B C 12 X X X Br2 X ✓ X Cl2 X ✓ ✓ - Identify the halide ions represented by letters A,B,and C (1 ½ mks)
- State the colour change for the reaction between chlorine and iodide ions and write an ionic equation for the reaction. (1 ½mks)
- In one of the dry practicals assignment to analyze cation a salt, the following observations were made:
Test Observation Inference (i) NaOH dropwise till in excess White ppt formed soluble in exces (ii) NH3 solution dropwise till in excess. Presence of Zn2+ ions confirmed. - Fill in the blanks in the table above. (2 mks)
- Give an ionic equation for the reaction that occurs in test (ii) when excess NH3 solution is added.
(1 mk)
- Describe how you would prepare a pure sample of lead (II) carbonate starting with lead (II ) oxide. (3 mks)
- Explain how sodium hydrogen carbonate and ammonium chloride are separated in solvey process.
(2 mks) -
- What is fuel? (1 mk)
- Firewood is the main source of fuel in most Kenyan homes. State two effects of wood products of burning fuel on environment. (1 mk)
- Calculate the mass of Calcium Oxide that can be obtained from 30g of calcium carbonate if completely decomposed by strong heating. (3 mks)
( Ca =40 , C=12, O=16) - State the two ions that causes hardness in water. (1 mk)
- Below is a table giving solubility a substance A and B at 20°C and 50°C
When aqueous mixture containing 55g of A and 12g of B at 80°C was cooled to 20°C crystals were formedSuubstance Solubility 100g of water 20°C 40°C A 40 65 B 15 17 - Identify the crystal formed (1 mk)
- Determine the mass of the crystals formed (1 mk
- Name the method use dot obtain the crystals (1 mk)
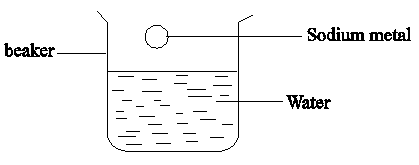
- The diagram below shows sodium metal being dropped in water. Study it and answer the questions that follow;
- State and explain two observations made during the reaction. (2 mks)
- Write an equation for the reaction that takes place during the experiment. (1 mk)
-
- In an experiment to determine solubility of solid P in water at 25°C, the following results were obtained.
Mass of empty evaporating dish – 24.2g
Mass of evaporating dish + saturated solution = 40.4g
Mass of evaporating dish + dry solid P = 28.4g
Using the information above calculate the solubility of solid Pat 25°C in g/100g of water. (2 mks) - State one precaution observed when carrying out the experiment in (i) above (1 mk)
- In an experiment to determine solubility of solid P in water at 25°C, the following results were obtained.
MARKING SCHEME
-
- Most reactive
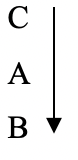 (2mks)
(2mks) - Carbonate C is soluble in water (1mk)
- Most reactive
-
- F (1mk)
- D1 (1mk)
- To Sodium sulphate add barium chloride if precipitate forms add dilute Hcl . If the substance is Na2 SO3 the precipitate dissolves. If the same treatment is given to Na2SO4 a white precipitate forms but insoluble in dilute HCl ( 3mk)
- A burning magnesium flame decomposes SO2 to form Sulphur and Oxygen. Oxygen reacts with Mg to form MgO. Wooden splint flame burns at a low temperature not hot enough to decompose SO2. (3mks)
-
- Candle goes off since all oxygen is used up✓.1
- Lime water forms white precipitate due to ✓1 the evolved from combustion of candle wax. (2mk)
-
- Water ✓1
- H – C Ξ C – H ✓1
- Polymerisation ✓1
- Heat to mixture ✓½ a beaker covered with a watch glass with water ½ in it. AlCl3 Sublimes ✓ ½ and deposits at the base of the watch glass. Sodium chloride remains ½ in the beaker as residue. ( 2mks)
- Salt J ✓1 . Its solubility increases with increase temperature ✓1. (2mks)
-
-
- L = 2.8.2 ✓½
- M= :2.7✓½ (1mk)
- LO ✓1
- Atomic radius of element M is less than ionic radius of M-,✓1 (1mk)
-
-
- Galvanization
- Copper is less reactive than ion. Copper will not corrode. Iron rust instead of copper.
-
- (C2H3)x = 59 ✓½
24 x 3x =54
27x = 54
X= 2. ⇒ ( C2H3)2
Molecular formula = C4 H6 ✓½ -
2 mentylpropane
(Any one of the above structures and name)
- (C2H3)x = 59 ✓½
- HOCl(aq) + Dye → HCL (aq) + dye [O] ✓1
H2SO3 + dye (O) → H2SO4 + dye ✓1 -
-
- Q ✓ ½
- R ✓ ½
-
-
-
- Produce stream ✓1
- Hydrogen ✓1
- Mg(s) + H2O(g) → MgO (s) + H 2 (g)✓1
- Moles of AlCl3 in 250 cm3
250 × 0.1 = 0.025✓1
1000
AlCl3 (aq) → Al3+(aq) + 3c ( 1am) ✓1
Moles of Al3+ in 250 cm3
0.0250 x 1 = 0.025 moles ✓½
Number of Al3+ = 0.025 x 6.0 x 1023
= 1.5 x 1022 ions ✓ ½ -
- T and V✓1
- T and V ✓1 Al (OH) 3 is amphteric hydroxide ✓1
-
- Graphite ✓1
- Diamond ✓1
- 20 cm3 of W takes → 12.6 sec
∴ 10cm3 of W → 10 × 12.6 = 6.3 seconds
20
0.56252 = Mw/32
Mw = 0.56252 x 32= 10.125g. ✓1
Accept any other method which is correct. -
-
- Argon is used in ore welding.
- Used in electrical light bulb
- Used in the production of titanium
- Used in growing of crystals of silicon and germanium.
( any threes uses 1mk each)
-
-
- A-Cl- ( Chloride Ion) ✓ ½
- B – I- ] ( Iodide Ion) ✓ ½
- C – Br‑ ( Bromide Ion) ✓½ ( 1 ½ mks)
- The solution turns from colourless to black ✓½ 2I (aq)+ Cl 2 (g)
 2C-Aq) + I 2 (aq)
2C-Aq) + I 2 (aq)
-
-
- Al3+; Zn2+, Pb2+ present✓1
White ppt formed soluble in excess✓1 - Zu (OH)2 (s) + 4NH3 (g)
 [Zu (NH3)4] 2+(aq) + 2OH-(aq) ✓1
[Zu (NH3)4] 2+(aq) + 2OH-(aq) ✓1
- Al3+; Zn2+, Pb2+ present✓1
-
- Measure 20cm3 of HNO3 and place it in a beaker.
- Add lead (II) Oxide until excess✓ ½
- Filter off the lead (II ) nitrate formed ✓1/2 and transfer the filtrate in a clean beaker.
- Add a solution of sodium carbonate to lead T ½ (II) nutrate a white precipitate forms which is lead (II) carbonate ✓2
- Filter off the precipitate and was it with distilled water than to dry the residue between dry filter paper.
- Separation is done by filtration ✓1 NaHCO3 is less soluble at low temperature .NH4cl is more soluble at low✓ temperatures. NaHCO3 crystal form and filtered off.
-
- Fuel is a substance which releases energy when burned. ( 1mk)
- Carbon in ✓½ firewood when burnt in insufficient air ✓½ forms CO✓. The gas is poisonous when
✓ ½ breathed in and leads to death carbon ( iv) Oxide is also formed. This gas can form acid rain and causes global warming. (2mks)
- Ca CO3(s)
 CaO(c) + CO2 (g)✓ 1
CaO(c) + CO2 (g)✓ 1
40 + 12 + 48 ✓½ 40+16 ½ 40+1 ✓½
100g 56g
If 100g of CaCO3 produce 56 g of Ca✓½ O
∴ 30g of CaCo3 (30 × 56) = 16.8g ✓½
(30 × 56) = 16.8g ✓½
100 -
- Calcium Ions ✓1
- Magnesium ✓1
-
- Substance A. ✓1
- 55 – 40 = 15g ✓1
- Fractional crystallization ✓1
-
-
- Hissing sound produced due to the production of hydrogen gas. ✓1
- Darts on the surface of water because hydrogen produced propels it. ✓1
- Melts into a silvery ball because of the heat produced during the reaction.
- Floats on the water because its less denser than water (mark any two correct)
- 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) ✓1
-
-
- Mass of solute = 28.4 – 24.2
= 4.2
Mass of water = 40.4 – 28.4 ✓1
= 12
Solubility = 4.2 x 100
12
= 35g/100gm ✓1 - Ensure that no solid is lost during evaporation ✓1
- Mass of solute = 28.4 – 24.2
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Sunrise Pre Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students