- 12.0cm 3 of 0.05M hydrochloric acid reacted completely with calcium hydrogen carbonate solution.
- Write the chemical equation for the reaction. (1 mk)
- Calculate the number of moles of hydrochloric acid used. (1 mk)
- Determine the number of moles of calcium hydrogen carbonate used. (1 mk)
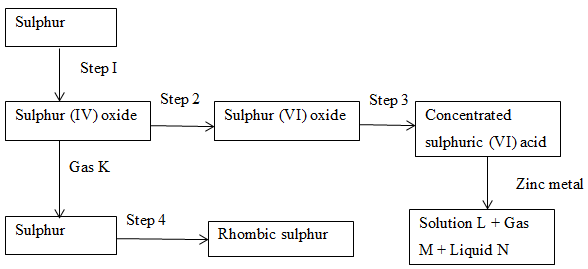
- Study the flow chart below and answer the questions that follow.
- Name
- Gas K. (1 mk)
- Gas M (1 mk)
- Steps 1, 2 and 3 constitute the contact process. State optimum conditions necessary for step 2 to occur. (1 mk)
- Explain what happens in step 4. (2 mks)
- Explain why water is not used in step 3 . (1 mk)
- Write an equation to show how pollution effects of sulphur (IV) oxide is controlled in contact process. (1 mk)
- State two uses of sulphur. (2 mks)
- Name
- Use the bond energies below to answer the questions that follow.
Determine the enthalpy for the following reactionBond Bond energy (KJ/Mol) H-H 436 C=C 612 C-C 347 C-H 413
C4H8(g) + H2(g) → C4H10(g) (3 mks) - Molar enthalpy of combustion of propanol is −1560Kjmol-1 .
- Write a thermochemical equation for the complete combustion of propanol. (1 mk)
- Calculate the amount of energy in joules released when 10g of propanol is burnt in excess oxygen. (C=12, H = 1.0,O=16.0) (2 mks)
-
- State Hess’ law (1 mk)
- Use the information below to answer the questions that follow.
C(s) + O2(g) → CO2(g) Δ Hθ = - 393Kjmol -1
H2(g) + ½O2(g) → H2O(l) ΔHθ = -287 Kjmol -1
C3H8 + 5O2(g) → 3CO2(g) + 4H2O(l) ΔHθ = -2209kj mol -- What does the symbol ΔHθ represent? (1 mk)
- Write the equation for the formation of propane from its constituent elements. (1 mk)
- With the aid of an energy cycle diagram, calculate the enthalpy of formation of propane. (3 mks)
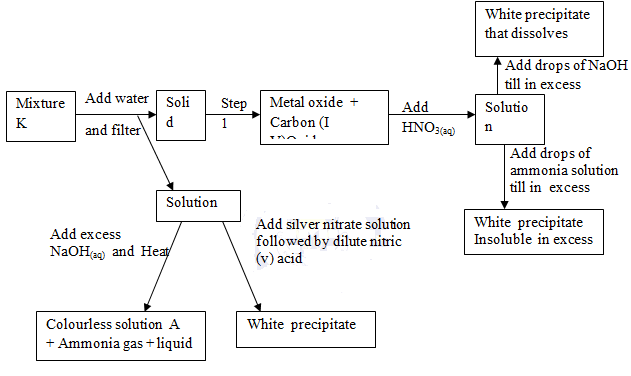
- Identify,
- Solution L: (1 mk)
- Solid M: (1 mk)
- Solution N: (1 mk)
- What condition is necessary in step 1 (1 mk)
- State the observations made in step 1 (1 mk)
- Write down an equation to show how;
- Solution N is formed. (1 mk)
- Colourless solution A and Ammonia gas are formed. (1 mk)
- When excess NaOH(aq) is added to solution N, a white precipitate is formed which dissolves. Give the name and formula of the ion formed. (2 mks)
- Name:……………………………………………………………………………………………….
- Formula: …………………………………………………………………………………………….
- Explain why it is necessary to add water to mixture K and then filter. (1 mk)
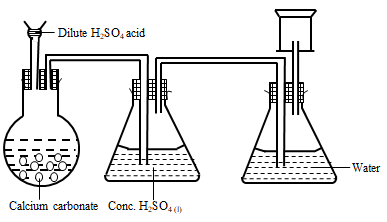
- State a correction for three mistakes in the set up above (3 mks)
- Give two reasons why carbon (IV) oxide is used as a fire extinguisher (2 mks)
- The flow chart below is for the manufacture of sodium carbonate by the Solvay process. Use it to answer the questions that follow.
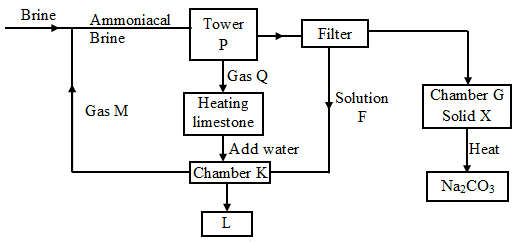
- Name gas
- M: ………………………….. (1 mk)
- Q: …………………………..
- Name solution F and solid X (1 mk)
- F: ………………………………………………………..
- X: ……………………………………………………….
- Name the product L formed and give one of its uses (2 mks)
- Write equations of the reactions in (2 mks)
- Tower:
- Chamber K:
- Name the two raw materials required in the manufacture of sodium carbonate (2 mks)
- Name gas
| Time (min) | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
| Mass (g) | 126.4 | 126.3 | 126.2 | 126.1 | 126.0 | 126 0 | 126.0 |
- Why is there a continuous loss of mass of the reaction mixture. (1 mk)
- Write an equation for the reaction taking place. (1 mk)
- State two different ways by which the reaction could have been made more rapid. (2 mks)
- Why does the mass remain constant after 8 minutes (1 mk)
- State the observations that would be made if a few drops of lead II nitrate solution was added to 1cm3 of the resulting solution followed by excess ammonia solution. (2 mks)
- State one environmental effect that excess carbon (IV) oxide in the air causes. (1 mk)
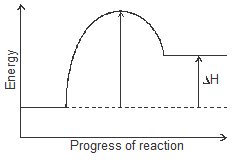
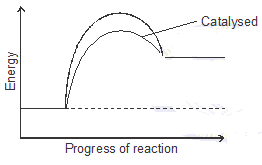
- The energy profile for the forward direction of a reversible reaction is shown.
Sketch on the diagram the path for a catalysed reaction. (1 mk) - What do you observe when you introduce the following substances in this equation
2CrO2-4(aq) + 2H+(aq) Cr2O2-7(aq) + H2O (l) ΔH= −477Kj/Mol
Cr2O2-7(aq) + H2O (l) ΔH= −477Kj/Mol
Yellow Orange- Dilute hydrochloric acid solution. Explain (2 mks)
- Increase heat. (2 mks)
MARKING SCHEME
-
-
-
- A and E / C and I √1 Any for 1mk
- D and E √1
-
-
-
- In gaseous form the compound lack mobile ions while in aqueous state it is in ionic form hence conduct electric current √1
-
- Melting point of oxide of H is higher than that of oxide of I;H oxide have Giant covalent structure with strong covalent bond √1 while oxide of I have simple molecular structure with weak intermolecular bond √1 2mks
- 4G + 3C2(g) → 2G2C3(s)
OR √
4Al(s) + 3O2(g) → 2Al2O3(s) √1
-
-
-
- Tetraammine copper (ii) ions √1
- Tetraammine zinc (II) ions √1
- Iron (III) hydroxide. √1
-
- 34g of KCl
 100g of H2O √ ½
100g of H2O √ ½
8.5g of KCl 8.5 × 100 of H2O √ ½
8.5 × 100 of H2O √ ½
34 - Mass = (34 – 27) √ ½
= 7g of KCl √ ½
- 34g of KCl
- When two solids are separately heated √ ½ and the gas passed over lime water, √ ½ white precipitate is formed √ ½ with sodium hydrogen carbonate while sodium carbonate does not produce a gas which turns lime water √ ½ into a white precipitate.
- Effevescences are seen in test tube with √ ½ water and no effervescence are seen in test tube √ ½ with methylbenzene. Water can react with magnesium to form hydrogen gas due to hydrogen ions √ ½ in water while methylbenzene is non – polar √ ½ hence lack ions.
-
- B; √ ½ requires less volume √ ½ of soap to form lather and the volume of soap used does not change even after boiling.
- Sample C contains temporary hardness √ ½ which is removed through boiling √ ½ hence reduction in volume of soap.
-
-
-
- 2HCl(aq) + Ca(HCO3)2(aq) → CaCl2(aq) + 2H2O(l) + 2CO2(g) √1
- 12.0 × 0.05 = 0.0006 moles √ ½ 1
1000 - 0.0006 √ ½ = 0.0003moles√ ½ 1
2
-
-
- Hydrogen sulphide √1
- Sulphur (IV) oxide √1
- Pressure of 2-3 atmosheres √ ½
Temperature of 450°c - Sulphur obtained from sulphur (IV) oxide is added to carbon (IV) sulphide in a boiling tube. The contents is then stirred and filtered √ ½ into a dry beaker. The filtrate is allowed to evaporate √ ½ to form Rhombic sulphur. 2
- When sulphur (IV) oxide is dissolved in water; excessive heat is generated that boil the acid to produce fine droplets that can cause burn. √1 1
- Ca(OH)2(aq) + SO2(g) → CaSO3(s) + H2O(l)
-
- Large scale manufacture of sulphuric (VI) acid √ 1
- Vulcanisation
- manufacture of bleaching agents
- Used as a fungicide.
- Manufacture of fireworks and dyes
-
-
-
- H H H H H H H
| | | | | | |
C = C – C – C – H + H – H H – C – C – C – C – H
H – C – C – C – C – H
| | | | | | | |
H H H H H H H H
(C=C) + 2( C-C ) + 8 ( C – H ) + H – H 3( C – C ) + 10 ( C – H )
3( C – C ) + 10 ( C – H )
612 + 2 x 347 + 8 x 413 3 x 347 + 10 x 413
3 x 347 + 10 x 413
612 + 694 +3304+ 436 1041 + 4130
5046 √1 5171 √1
5171 √1
∆H = -125KJmol- √1 3 -
- 2CH3CH2CH2OH(l) + 9O2(g) → 6CO2(g) + 8H2O(l). √1
- If 60g of C3H7OH
 1560KJ
1560KJ
10g of C3H7OH 10 × 1560 √1
10 × 1560 √1
60
= 260KJ √1
-
- The energy changes in converting reactants to products is the same regardless of the route by which the chemical change occurs. √1
-
- Standard enthalpy changes. √1
- 3C(s) + 4H2(g) → C3H8(g)
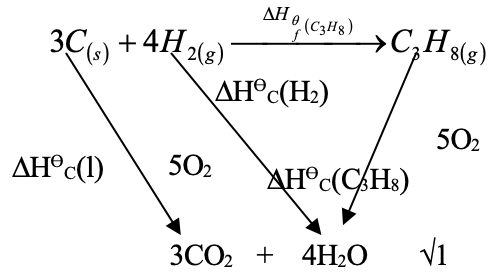
ΔHθf(C3H8) = 3ΔHθc(c) + 4ΔHθc(H2)) − ΔHθc(C3H8)
= ( 3 x – 393 + 4 x – 287) – ( − 2209) √1
=( - 1179 + - 1148) + 2209
= - 2327 + 2209
= - 118Kjmol-1 √1
- H H H H H H H
-
-
- NH4Cl/ Ammonium chloride √1
- PbCO3 (g) / Lead carbonate √1
- Pb(NO3)2 (aq) / Lead nitrate √1
- Heat √1
- Orange solid that cools to form a yellow solid √1
-
- PbOs + 2HNO3(aq) → Pb(NO3)2 + H2O(l) √1
-
- Name : Plumbate ion. √1
- Formula : (Pb(OH)4)2- √1
- Mixture K is made of both soluble and insoluble salts hence need to separate them. √1
-
-
-
- CO2 is collected by downward delivery. ✓1mk
- Exchange apparatus containing water and concentrated sulphuric (IV) acid. ✓1
- Use dilute hydrochloric acid for dilute sulphuric acid ✓
-
- It does not support combustion ✓1
- It is denser than air. ✓1
-
-
- M-Ammonia gas. ✓1/2
- Q-carbon (iv) oxide ✓1/2
-
- F-Ammonium chloride ✓1/2
- X-Sodium hydrogen carbonate. ✓1/2
-
- L-Calcium chloride ✓1
- Used as a drying agent ✓1
-
- Tower; P-NH3(aq)+CO2(g)+NaCl(aq)+H2O(l)
 NaHCO3(s)+NH4Cl(aq) ✓1
NaHCO3(s)+NH4Cl(aq) ✓1 - Chamber; K 2NH4Cl(aq) + Ca(OH)2(aq)
 CaCl2(aq) + 2NH3(g) + H2O(l) ✓1
CaCl2(aq) + 2NH3(g) + H2O(l) ✓1
- Tower; P-NH3(aq)+CO2(g)+NaCl(aq)+H2O(l)
-
- Sodium chloride. ✓1,
- Ammonia, coke or limestone. ✓1
-
-
-
- Due to production of CO2 which escape to the atmosphere 1
- CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g) 1
-
- Grind marble chips to powder form 1
- Increase concentration of HCl 1
- Increase the temperature of the reactants any 2 correct = 2mks
- The reaction is complete since calcium carbonate has been used up 1
- White precipitate ½ insoluble ½ in excess ammonia solution
-
- global warming
- cause acid rain any one = 1mk
-
-
- Favours forward reaction1orange colour intensify ✓1 concentration of hydrogen ions increases ✓1
- Favour backward reaction yellow colour formed✓1 the reaction produces heat/ exothermic✓1