INSTRUCTIONS TO THE CANDIDATE:
- Answer all questions in this paper.
- All working must be clearly shown.
- Mathematical tables and electronic calculators may be used.
-
- A Bunsen burner is a very important apparatus in the laboratory. Explain. (2 marks)
- Draw and name the apparatus you would use to measure exactly 250cm3 of a solution of sodium hydroxide. (2marks)
- The table below gives pH values of some solutions. Study it and answer the questions that follow.
Solution A B C D E pH value 7 5 14 12 1 - Select a solution that is likely to be carbonic acid (1mark)
- Identify solutions which would react with Zinc Oxide (1mark)
- Describe how you would separate a mixture of Barium chloride, Aluminium Chloride and Silver chloride. (3marks)
- Explain why the following substances cannot be used in the laboratory to prepare hydrogen gas
- Copper metal. (1mark)
- Nitric (v) acid (1mark)
- Lead metal (1mark)
- Write a balanced chemical equation for the reaction between sodium peroxide and water. (1mark)
- Study the table below then answer the questions that follow.
Particle Number of Protons Electrons Neutrons E 8 10 10 F 13 13 14 G 10 8 12 H 20 18 20 I 8 8 8 - Select from the table
- Neutral atom of a metal ( ½ mark)
- A pair of isotopes. Give a reason (1mark)
- A cation ( ½ mark)
- What is the charge on the cation identified in a(iii) above. ( ½ mark)
- What is the mass number of element G ( ½ mark)
- Illustrate the electron configurations of E. (2marks)
- Select from the table
-
- Define the term allotropes (1mark)
- Apart from carbon, name 2 other elements that show allotropy (1mark)
- Give the IUPAC names of the following compounds
- CH3 CH (CH3) C C CH3 (1mark)
- CH2 C(CH3) CH CH2 (1mark)
-
(1mark)
- A given mass of a gas occupies 20cm3 at 25°C and 650mmHg. Calculate the volume it will occupy at 10°C and 350mmHg (2marks)
- When anhydrous calcium chloride is exposed to the atmosphere, it forms a solution
- Name the process that takes place (1mark)
- State one use of the process displayed by anhydrous calcium chloride (1mark)
- In the equation below identify the reactant that acts as an acid. Give a reason for your answer (2marks)
N(aq) + H2O(l)NH3(g) + H3O+(aq)
- Explain the following observations
- Chlorine has a lower boiling point than lodine (1mark)
- Atomic radius of sodium is larger than that of phosphorous (1mark)
- Calculate the mass of 10 molecules of nitrogen gas (L=6.0x1023, N=14) (2marks)
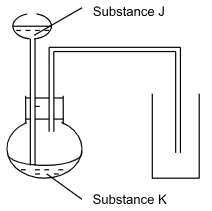
- The set-up below can be used to prepare some gases.
Complete the table by identifying substances J and K for the gases given below. (2marks)
Gas Substance J Substance K (i) Nitrogen (iv) Oxide (ii) Hydrogen Sulphide -
- State Gay Lussac’s law (1mark)
-
- Write an equation for complete combustion of methane (1mark)
- Calculate the volume of air (comprising 20% by volume of oxygen) that will be required for complete combustion of 200cm3 of methane. (2marks)
- Using dot(.) and cross(x) to represent electrons show bonding in carbon(II) Oxide. (1mark)
- Chlorine gas reacts with cold dilute potassium hydroxide to form a bleaching agent.
- Write an equation for the reaction (1mark)
- Give the name of the bleaching agent (1mark)
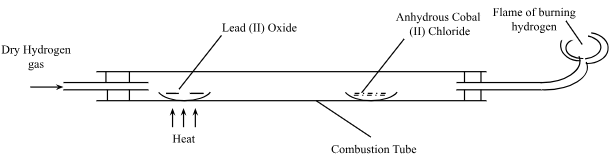
- Study the set-up below then answer the questions that follow.
- State the observations made on each of the following substances during the experiment
- Lead(II)oxide (1mark)
- Anhydrous cobalt (II) chloride (1mark)
- Name another gas that can be used in place of hydrogen in the experiment. (1mark)
- State the observations made on each of the following substances during the experiment
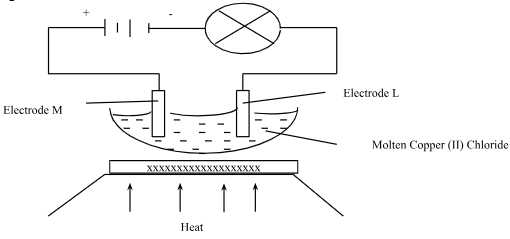
- The set-up below was used to show the products of electrolysis of molten copper (II) chloride using electrode
- What material are electrodes made of? (1mark)
- Which electrode is the cathode? Give a reason for your answer (1mark)
- State the observations made at electrode L (1mark)
- Write an Ionic equation for the reaction that took place at electrode M. (1mark)
- 0.2g of a compound containing carbon, hydrogen and oxygen on complete combustion gave 0.456g of Carbon (IV) oxide and 0.1862g of water. Calculate the empirical formula of the compound (C=12,H=1, 0=16) (3marks)
- Distinguish between Ionization energy and Electron affinity (2marks)
-
- Define the term molar enthalpy of formation. (1mark)
- Study the equations below then answer the questions that follow.
C(s) + O2(g)CO2(g) ΔH1= −393kJ/mole
H2(g) + ½ O2(g)H2O(l) ΔH2= −286 kJ/mole
C2H6 + 7/2O2(g)2CO2(g) + 3H20(l) ΔH3= −836kJ/mole
Using an energy cycle diagram calculate the enthalpy change for the formation of C2H6(g) (3marks)
- In terms of structure and bonding explain why ethanol is a liquid at room temperature while ethane is a gas. (2marks)
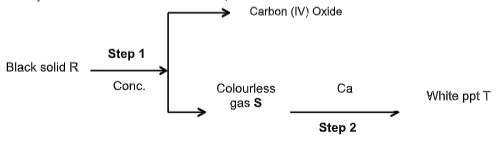
- Study the scheme below then answer the questions that follow.
- Name (3marks
- R ______________________________________________________
- S _______________________________________________________
- T _______________________________________________________
- Write a balanced chemical equation for the reaction that occurs in step I. (1mark)
- Name (3marks
- 4g of a metal carbonate XCO3 were dissolved in 160cm3 of 1M Hydrochloric acid. The resulting solution was then diluted to one litre. 25cm3 of this solution required 20cm3 of 0.1M sodium hydroxide for complete neutralization calculate.
- Number of moles of hydrochloric acid per litre that were in excess (2marks)
- Moles of the metal carbonate that reacted with the acid. (2marks)
- The atomic mass of metal X ( C=12, 0=16) (2marks)
- An element Y has an electron arrangement of 2.8.18.7
- To which group and period does the element belong. (1mark)
Group _______________________________________
Period ________________________________________ - Write the formula of the most stable ion of element Y (1mark)
- Compare the atomic and ionic radius of element Y. Explain (2marks)
- To which group and period does the element belong. (1mark)
- Given the following reagents: Barium carbonate, dilute Sulphuric (VI) acid, and any other suitable reagent describe how you would prepare a sample of barium Sulphate in the laboratory. (3marks)
- The following table shows some bond energies in KJ/mole use them to answer the questions that follows.
Calculate the energy change for hydrogenation 1 mole of ethane (3marks)Bond Energy(KJ/mole) C − C 348 C = C 611 H − H 436 C − H 414 - Concentrated sulphuric (vi) acid reacts with rock salt to form hydrogen chloride gas.
- Write an equation for the reaction (1mark)
- What property of Concentrated Sulphuric (VI) acid is demonstrated in the reaction above. (1mark)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions - Sukellemo Joint Pre Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students