Instruction to the candidates
- Answer all the questions in the spaces in the space provided in this paper using English.
- KNEC Mathematical tables and silent electronic calculators may be used.
- All working MUST be clearly shown where necessary
-
are isotopes of sodium
- Describe how these sodium isotopes are the same and how they are different in terms of the total number of protons, neutrons and electrons in each)
- same.(2mks)
- Different(1mk)
- Why do all three isotopes have an overall charge of zero?(1mk)
- Why do all three isotopes have the same chemical properties? (1mk)
- Why do sodium ions have a charge of +1? (1mk)
- Describe how these sodium isotopes are the same and how they are different in terms of the total number of protons, neutrons and electrons in each)
- Carbon is an element which exists in different forms.
- Name two forms of the element carbon that have giant covalent structures(1mk)
- Name the oxide of carbon that is a toxic gas.
-
- State Le Châteliers principle. ( 1mk)
- Explain how an increase in temperature affects the rate of a chemical reaction.(2mks)
- The reaction between ethane and oxygen is vigorous but the ethane needs to be ignited first. Explain. (2mks)
- During the reaction between excess magnesium and dilute hydrochloric acid the volume of hydrogen produced was recorded after every minute up to after three minutes after the reaction was over. A graph of volume of hydrogen produced was plotted against time in minutes.
- Describe the shape of the graph. (2mks)
- Explain how the rate of the reaction at the second minute can be determined using the graph. (2mks)
- Draw a set-up that can be used to carry out the above experiment. (3mks)
-
- The data below shows the solubility of potassium chlorate V in 100g water at different temperatures.
Solubility in g /100g water 8 10 14 20 40 Temperature (°C ) 25 31 38 52 88 - On the grid below plot a graph of solubility (y axis) against temperature(3mks)
- Determine the solubility of the solubility at 70°C (1mk)
- 400g saturated solution at 88°C is cooled to 10°C. Determine the mass of crystals obtained (3mks)
- A solution of ammonia in water is an electrolyte whereas a solution of ammonia in methylbenzene is not. Explain. (2mks)
- Copper(II) ions react with excess aqueous ammonia to form a complex ion.
- Write an equation for the reaction that forms the complex ion. (1mk)
- Name the complex ion. (1mk)
- Explain why CH4 is not acidic while HCl is acidic yet both compounds contain hydrogen(2mk)
- The data below shows the solubility of potassium chlorate V in 100g water at different temperatures.
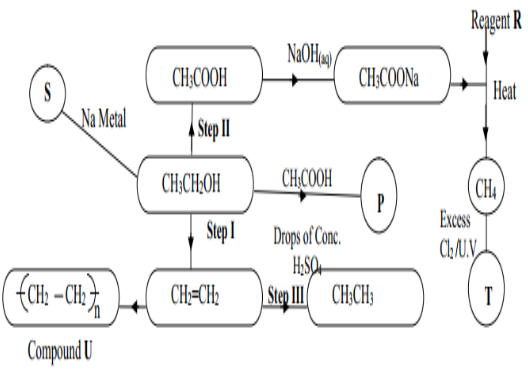
- Study the figure below and answer the questions that follow.
- Write the formula of the organic compounds P and S (2mks)
- Name the type of reaction, the reagent(s) and condition for the reactions in the following steps (4mks)
- Step I ______________________________________
- Step II ______________________________________
- Name reagent R___________________________________________________1mk)
- Draw the structural formula of T and give its name(2mks)
- Name compound U______________________________________ (.1mk)
- If the relative molecular mass of U is 42000, determine the value of n (C=12, H=1) (2mks)
- State why C2H4 burns with a more smoky flame than C2H6 (2mks)
-
- The enthalpies of ethane carbon and hydrogen combustion are -1250, -393 and -286 kJ/mol respectively.
- Draw an energy cycle diagram linking the heat of combustion of ethane carbon and hydrogen and the heat of formation of ethane.(2mks)
- Use the energy cycle to calculate the molar heat of the formation of ethane. .(2mks)
- Present the above information in an energy level diagram .(2mk)
- Calculate the enthalpy of formation of solid MOH given (3MKS)
M(S)+H2O(l) ? MOH(aq) + ½H2(g) −202kJ/mol
H2(g) + ½O2(g) ? H2O(l) −286kJ/mol
MOH(s) ? MOH(aq) −55kJ/mol - State two measures taken to reduce pollution by vehicles. (2mks)
- The enthalpies of ethane carbon and hydrogen combustion are -1250, -393 and -286 kJ/mol respectively.
-
- The heat of solution of magnesium chloride is −180kJ/mol. . given that the lattice energy of magnesium chloride is 2493kJ/mol and the hydration energy of magnesium is −1891kJ/mol.
- Draw an energy cycle diagram to show this information. (2mks)
- Calculate the hydration energy of a chloride ion. (2mks)
- Draw an energy level diagram to represent the above information. (3mks)
- starting with glucose describe how absolute ethanol can be prepared. (4mks)
- Starting with dodecene describe how the soapless detergent sodium alkylbenzene sulphonate can be prepared.(4mks)
- The heat of solution of magnesium chloride is −180kJ/mol. . given that the lattice energy of magnesium chloride is 2493kJ/mol and the hydration energy of magnesium is −1891kJ/mol.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions - Sukellemo Joint Pre Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students