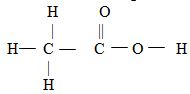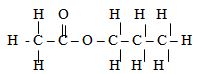Instructions to candidates
- Answer all the questions in spaces provided .
- All working MUST be clearly shown.
- KNEC mathematical tables and silent non programmable electronic calculators may be used.
- Candidates should answer the questions in English
-
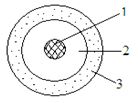
- Draw a labeled diagram showing atomic structure of (23 11 Na.) 2mks
- The atomic number of phosphorus is 15.Draw a dot (•) and cross(x) diagram for the compound formed when phosphorus reacts with hydrogen atomic number 1 (1mk)
- Study the following heat changes and answer questions that follow
NaCl(s) → Na+(g)+Cl-(g) ∆ H1 +781kJmol-
Na+(g) → Na(aq) ∆H2 -390kJmol-
Cl-(g) → Cl-(aq) ∆H3 -384kJmol-- Identify the heat changes (1MK)
H1
H2 - Calculate the heat of solution of sodium chloride using the above heat changes (2mks)
- Identify the heat changes (1MK)
- Dry carbon (II) oxide gas reacts with heated lead(II)oxide as shown in the equation below.
PbO(s) + CO(g) Pb(s) + CO2(g)- Name the process undergone by the lead(II)oxide. (1mk)
- Give a reason for your answer in (a) above. (1mk)
- Name another gas that can be used to perform the same function as carbon(II)oxide gas in the above reaction. (1mk)
- The following reaction is in equilibrium in a closed container
2SO2(g) + O2(g) ⇌ 2 SO3(g) ∆H = -Ve
State giving reasons how an increase in temperature would affect the amount of sulphur (VI) oxide gas. (2mks) - The standard electrode potential for elements P, Q, R and S are given below. Eɵ (volts)
P2+ (aq) + 2e- → P(s) -2.40
Q2+ (aq) + 2e- → Q(s) -0.80
2R+ (aq) + 2e- → R2(g) 0.00
S2+ (aq) + 2e- → S(s) +0.35
½ T2 (aq) + e- → T-(aq) +1.40- What is the Eɵ value for the strongest oxidizing agent? (1mk)
- Which two of the above elements in an electrochemical cell produce the largest e.m.f . (1mk)
- Calculate the electromotive force of the cell in (b) above. (1mk)
-
- What is meant by the term:
Half-life:- (1mk) - State one application of radioactivity in medical
- 50g of a radioactive substance was reduced to 6.25g in 36.3years. Calculate the half-life of the substance. (2mks)
- What is meant by the term:
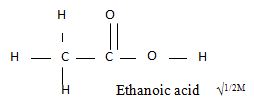
- The structure below belongs to a member of alkanoic acid.
- Give the name of the Structure. (1mk0
- What is the total number of electrons used for bonding in a molecule of the structured (2mks)
Named in (a)
- The purple color of a solution containing manganese (vii) ions disappears when iron (ii) ions are added. The ionic equation for the reaction which occurs is;
MnO-4(aq) + 5Fe2+(aq) + 8H+(aq) → Mn2+ (aq) + 5Fe3+ (aq) + 4H2O (l)
With reasons state which substance is acting as a; (2 mks)- Reducing agent.
- Oxidizing agent
- 3.1g of an organic compound containing carbon ,hydrogen and oxygen only produced 4.4g of carbon(iv) and 2.0 of water on combustion
- Calculate its empirical formulae (2mk)
- Calculate its molecular formular if its mass 90. (1mk)
- Esters, fats and polyesters all contain the ester linkage. The structural formula of the ester is given below.
Name two chemicals that could be used to make this ester and draw their structural formulae. Show all bonds. (2mks) - An iron sculpture was produced to commemorate the anniversary of founder of a certain village. To prevent it from rusting, the village elder attached it by a wire to a block of zinc which was stored underground out of sight.
- Explain how the village elder’s action would prevent the rusting of the sculpture. (1mk)
- What name is given to this method of preventing rusting? (1mk)
- List down two other ways in which rusting of the statue could be prevented. (1mk)
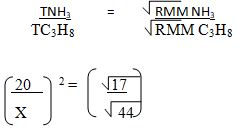
- 50cm3 ammonia gas diffuses through a small orifice in 20 seconds. How long will it take a similar volume of propane (C3H8) to diffuse through the same orifice under the same conditions of temperature and pressure? (C=12.0, H =1.0, N=14.0) (3mks)
-
- What observations would be made if hydrogen sulphide gas was bubbled through a solution of Copper (II) sulphate. (1mk)
- Write an equation for the reaction that takes place in (a) above. (1mk)
- Chlorine reacts with methane as shown below.
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
What condition is necessary for this reaction to take place? (1mk)
- The table below gives some properties of three metals: Aluminium, iron and copper. Use it to answer the questions that follow.
Metal Density Tensile Strength 1010pa Electrical conductivity Aluminium 2.70 7.0 0.38 Iron 7.86 21.1 0.10 Copper 8.92 13.0 0.59
Assuming that steel and stainless steel have similar properties to iron.- Why do some stainless steel sauce pans have a copper base? (1mk)
- Aluminum with a steel core is used for overhead power cables in preference to copper. Why is aluminium preferred ? (1mk)
- Apart from over head power cables copper is chosen for almost all other electrical uses.
Suggest two reasons for the choice of copper. (2mks)
- A form four student wanted to determine the solubility of potassium nitrate. He obtained the following results.
Mass of evaporating dish = 15.13g
Mass of evaporating dish and solution. = 36.51g
Mass of evaporating dish and salt = 19.41g
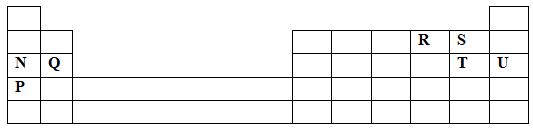
Use the information above to calculate the solubility of potassium nitrate. (3mks) - The grid below is part of the periodic table. Use it to answer the questions that follow. ( The letters do not represent the actual symbols of elements.)
- Indicate in the grid the position of an element represented by letter V, whose atomic number is 14. (1mk)
- Select a letter which represents a monoatomic gas. (1mk)
- Write an equation for the reaction between Q and T (1mk)
- The table below shows ammeter readings recorded when two equimolar solutions were tested separately.
Electrolyte Current (A) Dilute Sulphuric (VI) Acid 7.2 Ethanoic Acid 4.0
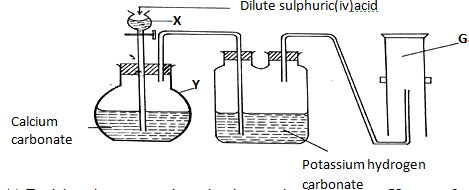
Explain the difference in the ammeter readings. (1mk) - A student set up the apparatus for the preparation of carbon (IV) oxide gas as shown below.
Study the set up and answer the questions that follow.
- Explain using an equation why the reaction in apparatus Y occurs for a very short time then stops. (1mk)
- What is the purpose of passing the gas through potassium hydrogen carbonate? (1mk)
- State and explain why there is no sample of carbon (IV) oxide gas collected. (1mk)
- Describe how a solid of ammonium sulphate can be prepared starting with 100cm3 of 2M ammonium hydroxide (3mks)
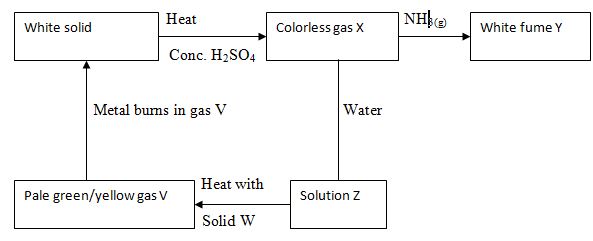
- Study the scheme below and answer the questions that follow.
- Write an equation for the formation of white fumes Y. (1 mk)
- What is the function of solid W in the reaction? (1 mk)
- Identify gas V. (1 mk)
- Explain the effect of the following on the rate of reaction in terms of the collisions theory ;(3mks)
- Increase in concentration
- Change in pressure
- Use of catalyst
- Explain each of the following observations.
- Soft drinks fizz when the cap is removed from the bottle. (1mk)
- Diamond does not conduct electricity while graphite does. (1mk)
- Pure nitric (V) acid is colourless but during its laboratory preparation, it appears yellow. (1mk)
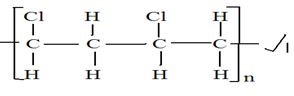
- Polyvinyl/ chloride (PVC) is an example of an addition polymer whose monomer is Chloroethene.
- What is a polymer? (1mk)
- What is meant by addition polymerization? (1mk)
- Using 2n molecules draw the structure of PVC. (1mk)
-
- State Boyles’s law (1mk)
- A gas occupies 500cm3.at 27ċ and 100,000pa. What will be its volume at 0ċ and 101325pa (2mks)
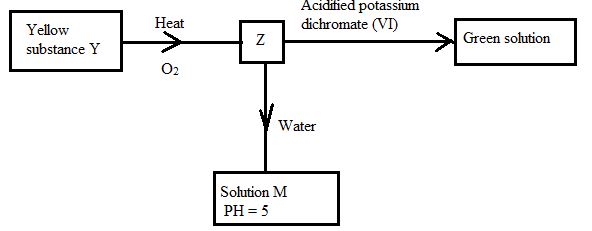
- Study the flow chart below and answer the questions that follow.
Identify (3mks)
Z
M
Y - The diagram below represents pipes used in Frasch pump for the extraction of sulpur
Which substance passes through tube? (3mks) - In the last stage of the solvay process, a mixture of sodium hydrogen carbonate and ammonium chloride is formed.
- State the method of separation used. (1mk)
- Write an equation showing how lime is slaked (1mk)
- Name the by - product recycled in the above process. (1mk)
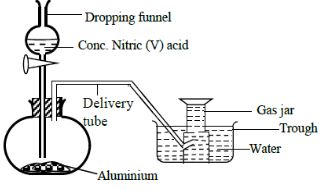
- In order to prepare hydrogen gas in the laboratory a student set-up the apparatus shown in the diagram below. Study it and answer the questions that follow.
- Suggest why the student did not collect hydrogen gas. (1mk)
- In a separate experiment the student reacted iron and hydrochloric acid to prepare hydrogen gas.
- Write an ionic equation for the reaction. (1mk)
- The hydrogen gas produced was found to have a foul smell. Suggest an explanation for this. (1mk)
MARKING SCHEME
- (a)
-
-
- H1 Lattice Energy √½
- H2 Hydration Energy √½
- -774+781=+7kJ/mol 2
⇒ (414 x 4) + 244 = 1900kJ✔ 1
-
-
- reduction √1
- Removal of oxygen gas from a substance is reduction √1
OR - Lead ions gained electrons to become lead metal and gain of electrons is a reduction √½ - hydrogen✔1
- O2 Lowered or reduced. √1mk increasing temperature, equilibrium shifts to the left since forward reaction is exothermic. √1mk
- -2.40V
- P and T2 1m
- Eθ = +1.4 - -2.40 (1mk)
= +3.8V
-
- Half life is the time required for the initial number of atoms/mass of a radio isotope to decay
- Destroy cancerous tissues in patient or sterilization of surgical instruments or iodine 131 used in patient with defective thyroid or monitor growth in bones and healing of fractures or provide heat pace setters .
- t ½ (half life) 50g 25g 10.5 6.25
∴there are 3 half lifes)
3 t ½ = 36.3 years (√1mk)
t ½ = 36.3
3
t ½ = 12.1; years (√1mk)
-
- Ethanoic acid. (√1mk)
- 16 electrons (√1mk)– single bonds constitute 2 electrons double 4 electrons (√1mk)
OR structure has 8 electrons and bond has e electrons.(2mks)
-
-
- Fe2+√½
- donates an electron√1/2/ there is increase in oxidation state from 2+ to 3+ (Fe2+ to Fe3+)
- MnO-4√½
- gains electrons√½/ there is decrease in oxidation state from 7+ to 2+ (Mn7+ to Mn2+)
- Fe2+√½
-
-
- C =4.4/44*12=1.2
- H=2/18*2=0.2 ½MK
- O=3.1-1.4=1.7
1M
C H 0 1.2 0.2 1.7 12 1 16 0.1 0.2 0.106 1 2 1
CH2O ½M
C3H6O3 1M
-
propanol -
- Zinc is more√½ reactive than iron, hence it is going to corrode√½ instead of iron
- Sacrificial protection √1
-
- painting✔1
- Alloying
- Oiling and greasing
(Any two one mark each ½ mark
-
X = 32.18seconds √(1mk) -
- Black precipitate is formed √(1mk)
- H2S(g) + CuSO4(aq) H2SO4(aq) + CuS(s) √(1mk)
- U.V light
-
- Copper has higher thermal conductivity than stainless steel. √(1mk)
- Since steel has higher tensile strength than aluminium hence greater resistance to breaking. √(½mk)
-
- It is less costly / cheaper √(1mk)
- Less high electrical conductivity √(1mk)
Mass of solution = 36.51 – 15.13 = 21.38
Mass if salt = (19.4 – 15.13)g = 4.28g
Mass of water = mass of solution – mass of salt (21.38 – 4.28)g = 17.1g of water
17.1g of water dissolve 4.28g of salt (KNO3)
100g of water = (100 x 4.28)g 100g H20)
17.1
= 25.0292g/100g H2O)-
- period 3 group 4√(1mk)
- U√(1mk)
- Q(s) + T2(g) → QT2(s) √(1mk)
- Dilute Sulphuric (VI) acid has a higher value √ ½ than ethanoic acid because it is a stronger √½ acid and fully √½ ionizes in water than ethanoic acid that only partially ionizes. √ ½
-
- White insoluble ppt. forms that coats the reactants stopping further reaction. √½
CaCO3(s) + H2SO4 (aq) → CaSO4(s) + CO2 (g) + H2O (l) (both √) - To remove H2SO4/acid fumes √½
- Wrong choice of reagents reacted
- White insoluble ppt. forms that coats the reactants stopping further reaction. √½
-
-
- To 100cm3 of 2M ammonium hydroxide add 50cm3 0f 2M or 100cm3 0f 1M sulphuric(vi0acid (1mrk)
- Heat to saturate ,cool for the crystals to form then dry the crystals between two filter papers (2mrk)
-
-
- NH3(g)+HCl(g) NH4Cl(s)
- Oxidising agent
- Chlorine/Cl2
-
- Increase in concentration increases the number of the particles in the system. When the concentration of particle of the reactant is high , the number of collions is also high. This increases the number of successful collions and increases in the rate of reactions. (1mrk)
- Increase in pleasure increases the number of particles in the system therefore the rate increases . reduced pressure results in the number of collions and hence reduces rate of reactions. (1m)
- Use of a catalyst lowers the activation energy of the reacting particles therefore more particles attain activation hence increases rate of reactions 1mk
-
- Soft drinks contain carbon IV oxide under pressure which is released on opening ✔1m
- In diamond, all the four valence electrons are used in bonding hence no delocalized electrons ✔ ½ while in graphite one of the four valence electrons is not used in bonding hence delocalized for conduction ✔ ½
- Heat decomposes some nitric V acid to nitrogen IV oxide which dissolves in the acid making the acid appear yellow ✔1
-
- A polymer is a long-chain molecule made by joining many small molecules ✔1
- A process where unsaturated small molecules join to form long chain molecules without
Formation of any other products. ✔1 -
-
- The volume of a fixed mass of a gas is inversely proportional to its pressure at constant temperature 1mk
- p1vi/t1=p2v2/t2 ½mk
(100000*500*273)/(101325*300) (1m) = 449cm3 0r 449.05cm3 ½mk
-
- Z-sulphur(iv)oxide✔1
- M-sulphurous acid✔1
- Y-sulphur✔1
-
- Compressed hot air, in ✔1
- Molten froth of Sulphur water mixture, out ✔1
- Superheated water – in at a temperature of 170ċ ✔1
-
- Fractional crystallization✔1
- CaO + H2O → Ca (OH)2 ✔1
- Ammonia gas ✔1
-
- Conc. HNO3 is a strong oxidizing agent. It oxidizes and thickens the thin coating of aluminium oxide thus leading to no reaction between metal and acid. ✔1
- Fe(s) + 2H+ (aq) → Fe2+ + H2 (g) ✔1
- Sulphide impurities in the iron react with the acid to form hydrogen sulphide gas which has foul smell. ✔1
Download Chemistry Paper 1 Questions and Answers - Lugari Constituency Joint Pre Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students