-
- Study the information in the table below and answer the questions that follow. The letters do not represent the actual symbols of the elements.
Ionisation element Electron configuration Energy Kj/mol P 2.1 519 Q 2, 8, 1 494 R 2.8.8.1 418 - What is the general name given to the group in which elements P, Q and R belong? (1 mk)
- What is meant by Ionisation energy? (1 mk)
- Explain why element Pl has the highest ionization energy. (2 mks)
- When a piece of element Q is placed on water, it melts and a hissing sound is produced as it moves on the surface of the water. Explain this observation. (3 mks)
- Write an equation for the reaction between element Q and water. (1 mk)
- Neutralization is one of the methods of preparing salts.
- What is meant by neutralization? (1 mk)
- Describe how you would prepare crystals of Sodium nitrate starting with 200cm3 of 2M Sodium hydroxide. (3 mks)
- Write an equation for the reaction that takes place when a solid sample of Sodium nitrate is heated. (1 mk)
- Study the information in the table below and answer the questions that follow. The letters do not represent the actual symbols of the elements.
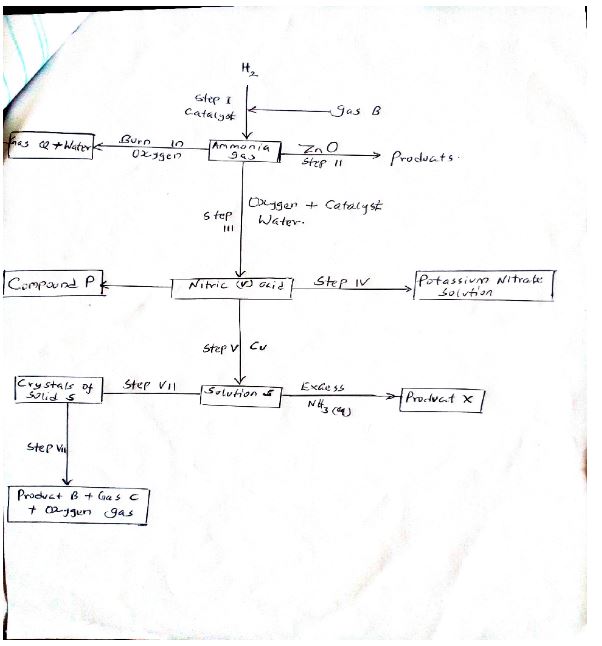
- Study the flow chart below and answer the questions that follow.
- State one source of gas B. (1 mk)
- Name the catalysts used in:- (2 mks)
- Step I
- Step III
- Write chemical equations for reactions in; (2mks)
- Step II
- Step IV
- Name the process that takes place in; (2mks)
- Step (IV)
- Step VI
- Name and write the chemical formula of product X (2mks)
Name:
Formula - State one laboratory use of compound P (1mk)
- Identify any other gas that can be used instead of ammonia in step II (1mk)
- State one use of gas Q (1mk)
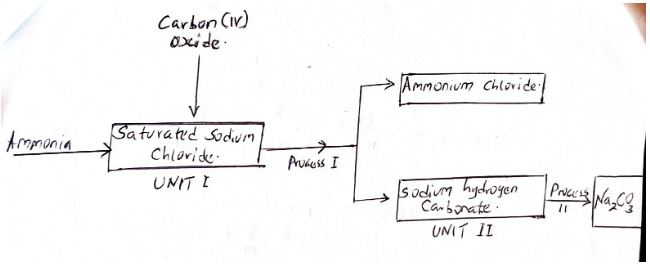
- The scheme below shows part of the Solvay process used for the manufacture of sodium carbonate.
-
- Explain how sodium Chloride required for this process is obtained from sea water. (2mks)
- Two main reactions takes place in UNIT I. The first one is the formation of
Ammonium hydrogen carbonate.- Write an equation for this reaction. (1mk)
- Write an equation completely with 40.0cm3 of 0.5 M Sulphuric (VI) acid for the second reaction (1mk)
- State how the following are carried out. (2mks)
- Process I
- Process II
- In an experiment to determine the percentage purity of the sample of sodium Carbonate produced in the Solvey process, 2.15g of the sample reacted completely with 40.0cm3 of 0.5 M Sulphuric (VI) acid.
- Calculate the number of moles of sodium carbonate that reacted. (2mks)
- Determine the percentage of Sodium Carbonate in the sample. (Na=23, C=12, O=16. (2mks)
- Name two industrial uses of Sodium Carbonate. (2mks)
-
-
- Give the name of each of the processes described below which takes place when the salts are exposed to air for sometimes.
- Anhydrous copper sulphate become Wet. (1mk)
- Magnesium Chloride from an aqueous solution (1mk)
- Fresh crystals of sodium Carbonate, Na2CO3. 10H2O, become covered with a white powder of formula, Na2CO3. H2O (1 mk)
- Write the formula of the complex Ion formed in each of the reactions described below.
- Zinc metal dissolves in excess ammonia solution (1mk)
- A hydrated salt has the following composition by mass Iron 20.2%, Oxygen 23.0%, Sulphur 11.5%, water 45.3%. Its relative formula mass is 273.
- Determine the formula of the hydrated salt. (fe=56, S=32, O=16, H=1) (3mks)
- 6.95g of the hydrated salt were dissolved in distilled water and the total volume made to 250cm3 of solution. Calculate the concentration of the salt solution in mole per litre. (2mks)
- Give the name of each of the processes described below which takes place when the salts are exposed to air for sometimes.
-
-
- In the space provided sketch a labeled diagram to show how hydrogen chloride gas can be prepared and collected in the laboratory using Sodium Chloride and concentrated Sulphuric (VI) acid (the gas needed not to be dry). (3mks)
- Write an equation for the reaction that takes place. (1mk)
- Name one drying agent for hydrogen Chloride. (1mk)
- State and explain the observation that would be made when hydrogen Chloride gas is bubbled through a solution of lead (ii) Nitrate. (3mks)
- A sample of hydrogen Chloride gas was dissolved in water to make 250cm3 of solution. 25cm3 of the solution required 46cm3 of 11.0M Sodium hydroxide for complete neutralization.
- Calculate the number of moles of hydrochloric acid in 25cm3 of solution. (2mks)
- Determine the mass of hydrogen Chloride that was dissolve to make 250cm3 of solution. (Cl=35.5, H=1.0) (3mks)
-
- In an experiment, a piece of Magnesium ribbon was cleaned with steel wool. 2.4g of the clean Magnesium ribbon was placed in a crucible and completely burnt in Oxygen. After cooling, the product weighed 4.0g.
- Explain why it was necessary to clean the Magnesium ribbon. (1mk)
- What observation was made in the crucible after burning? (1mk)
- Why was theme an increase in mass? (1mk)
- Write the equation for the reaction which took place in the crucible (1mk)
- The product in the crucible was shaken with water and filtered. Explain the observation which was made when blue and red litmus papers were dropped into the filtrate. (2mks)
- Calculate the volume of oxygen gas used during the burning. (O=16, Mg= 24, Molar volume of the gas = 24000cm3 at room temperature) (3mks)
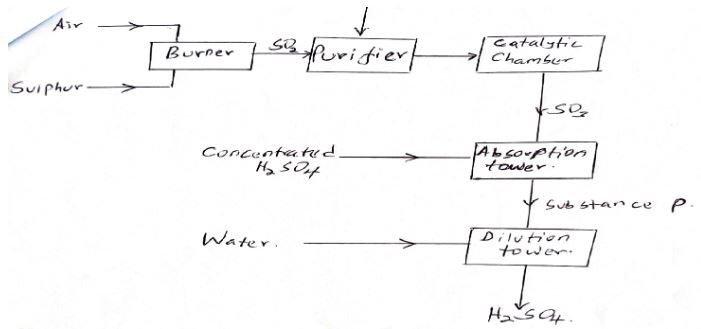
- The flow chart below shows the large scale manufacture of Sulphuric acid.
- Why is the purifier necessary? (1mk)
- Name the catalyst used in contact process. (2mks)
- Name and give the formula of substance P (1mk)
- Write the equation for the formation of substance P. (1mk)
- State the optimum temperature and pressure for the contact process.
- Pressure. (1mk)
- Temperature (1mk)
- Sulphur (IV) Oxide emitted in this process undergoes process known as scrubbing. What is scrubbing? (3mks)
- When compound P is heated with concentrated Sulphuric acid, a gas which forms dense white fumes with ammonia is liberated.
- Identify the anion in P. (1mk)
- Write an ionic equation for the reaction between a solution of P and silver nitrate solution. (1mk)
- State what would be observed if lead (II) Nitrate solution was added to a solution of P and mixture heated. (2mks)
MARKING SCHEME
- Study the information in the table below and answer the questions that follow. The letters do not represent the actual symbols of the elements.
Ionisation element Electron configuration Energy Kj/mol P 2.1 519 Q 2, 8, 1 494 R 2.8.8.1 418 - What is the general name given to the group in which elements P, Q and R belong? (1 mk)
- Alkali metals
- What is meant by Ionisation energy? (1 mk)
- It is the minimum amount of energy required by an atom to lose the outermost electron in a gaseous state.
- Explain why element P has the highest ionization energy. (2 mks)
- Its outermost electrons is close to the nucleus hence held strongly to the nucleus.
- When a piece of element Q is placed on water, it melts and a hissing sound is produced as it moves on the surface of the water. Explain this observation. (3 mks)
- It melts because of the heat produced since the reaction is Exothermic.
- The hissing sound is due to the production of hydrogen gas during reaction, it moves on the surface due to its being propelled by the hydrogen gas.
- Write an equation for the reaction between element Q and water. (1 mk)
- 2Ca + 2H2O 2QOH (aq) + H2 (g)
- What is the general name given to the group in which elements P, Q and R belong? (1 mk)
- Neutralization is one of the methods of preparing salts.
- What is meant by neutralization? (1 mk)
- It is the reaction between an acid and base to form a salt and water only.
- Describe how you would prepare crystals of Sodium nitrate starting with 200cm3 of 2M Sodium hydroxide. (3 mks)
- Add 200cm3 of 2M nitric (V) acid to 200cm3 of 2m Sodium hydroxide.
- Write an equation for the reaction that takes place when a solid sample of Sodium nitrate is. heated (1 mk)
- 2NaNo3 2NaNo2 + O2
- What is meant by neutralization? (1 mk)
- Study the information in the table below and answer the questions that follow. The letters do not represent the actual symbols of the elements.
- Study the flow chart below and answer the questions that follow.
- State one source of gas B. (1 mk)
- Fractional Distillation of liquid air
- Name the catalysts used in:- (2 mks)
- Step I
- Finely divided iron
- Step III
- Platinum – rhodium
- Step I
- Write chemical equations for reactions in; (2mks)
- Step II
2NH3 + 3ZnO 3Zn(s) + N2(g) +3H2(g - Step IV
2Cu(NO3)2 → 2CuO(s) + 4NO2(g) + O2(g)
- Step II
- Name the process that takes place in; (2mks)
- Step (IV)
- Neutralisation
- Step VI
- Crystallization
- Step (IV)
- Name and write the chemical formula of product X (2mks)
Name:
Tetra amine copper (ii) Ion
Formula (Cu (NH3)4)2+ - State one laboratory use of compound P (1mk)
- Preparation of nitrogen (i) Oxide gas
- Identify any other gas that can be used instead of ammonia in step II (1mk)
- Hydrogen
- State one use of gas Q (1mk)
- Manufacture of ammonia gas through the Haber process
- State one source of gas B. (1 mk)
- The scheme below shows part of the Solvay process used for the manufacture of sodium carbonate.
-
- Explain how sodium Chloride required for this process is obtained from sea water. (2mks)
- Channel or pump sea water into shallow ponds.
- Evaporation of water occur at the ponds and sodium chloride crystallizes out.
- Two main reactions takes place in UNIT I. The first one is the formation of Ammonium hydrogen carbonate.
- Write an equation for this reaction. (1mk)
- NH3(g) + CO2(g) + H2O NH4HCO3
- Write an equation completely with 40.0cm3 of 0.5 M Sulphuric (VI) acid for the second reaction (1mk)
- NH4HCO3(aq) + Nacl (ag) NaHCO3(s) + NH4Cl(aq)
- Write an equation for this reaction. (1mk)
- State how the following are carried out. (2mks)
- Process I
- filtration
- Process II
- Heating
- Process I
- In an experiment to determine the percentage purity of the sample of sodium Carbonate produced in the Solvey process, 2.15g of the sample reacted completely with 40.0cm3 of 0.5 M Sulphuric (VI) acid.
- Calculate the number of moles of sodium carbonate that reacted. (2mks)
Na2CO3(s) + H2SO4(ag) Na2SO4(aq) + CO2(g) + H2O (l)
Moles = of H2SO4 = 40 x 0.5 == 0.02 moles= Moles of Na2Co3 - Determine the percentage of Sodium Carbonate in the sample. (Na=23, C=12, O=16. (2mks)
Mass of Na2CO3 = 0.02 x 106= 2.12g
Percentage purity = 2.12x 100 = 98.6%
2.15
- Calculate the number of moles of sodium carbonate that reacted. (2mks)
- Explain how sodium Chloride required for this process is obtained from sea water. (2mks)
- Name two industrial uses of Sodium Carbonate. (2mks)
- Manufacture of glass
- Textile industries
- Softening hard water
- Making of detergents
-
-
- Give the name of each of the processes described below which takes place when the salts are exposed to air for sometimes.
- Anhydrous copper sulphate become Wet. (1mk)
- Hygroscopy
- Magnesium Chloride from an aqueous solution (1mk)
- Deliquescence
- Fresh crystals of sodium Carbonate, NA2CO3. 10H2O, become covered with a white powder of formula, Na2CO3. H2O
- Efflorescence
- Anhydrous copper sulphate become Wet. (1mk)
- Write the formula of the complex Ion formed in each of the reactions described below.
- Zinc metal dissolves in excess ammonia solution (1mk)
(Zn(NH3)4)2+
- Zinc metal dissolves in excess ammonia solution (1mk)
- A hydrated salt has the following composition by mass Iron 20.2%, Oxygen 23.0%, Sulphur 11.5%, water 45.3%. Its relative formula mass is 273.
- Determine the formula of the hydrated salt. (fe=56, S=32, O=16, H=1) (3mks)
Fe S O H2O 20.2
5611.5
3223.0
1645.0
180.3 0.36 1.44 2.52 42 1 4 7
Empirical mass (56+32+64+7(18)=278
Formula F2SO4 . 7H2O - 6.95g of the hydrated salt were dissolved in distilled water and the total volume made to 250cm3 of solution. Calculate the concentration of the salt solution in mole per litre. (2mks)
6.95 = 0.025 moles thus 100 x 0.025
278 200 0.1M
- Determine the formula of the hydrated salt. (fe=56, S=32, O=16, H=1) (3mks)
- Give the name of each of the processes described below which takes place when the salts are exposed to air for sometimes.
-
-
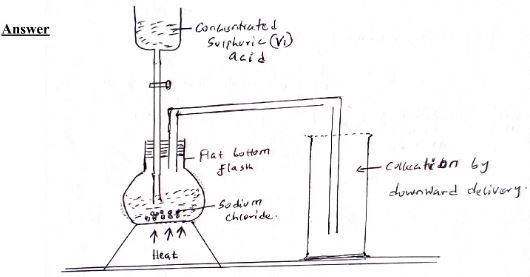
- In the space provided sketch a labeled diagram to show how hydrogen chloride gas can be prepared and collected in the laboratory using Sodium Chloride and concentrated Sulphuric (VI) acid (the gas needed not to be dry). (3mks)
- Write an equation for the reaction that takes place. (1mk)
- Nacl(s) + H2SO4(aq) NaHSO4 (aq) + HCL (g)
- Name one drying agent for hydrogen Chloride. (1mk)
- Concentrated Sulphuric (VI) acid or Anhydrous Cacl2
- State and explain the observation that would be made when hydrogen Chloride gas is bubbled through a solution of lead (ii) Nitrate. (3mks)
- A white precipitate of Pbcl2 is produced. HCl gas in water ionizes to form H+ ions, Cl- ions, the CL- ions combine with Pb2+ ions to form Pbcl2 which is insoluble
- In the space provided sketch a labeled diagram to show how hydrogen chloride gas can be prepared and collected in the laboratory using Sodium Chloride and concentrated Sulphuric (VI) acid (the gas needed not to be dry). (3mks)
- A sample of hydrogen Chloride gas was dissolved in water to make 250cm3 of solution. 25cm3 of the solution required 46cm3 of 11.0M Sodium hydroxide for complete neutralization.
- Calculate the number of moles of hydrochloric acid in 25cm3 of solution. (2mks)
HCl (aq) + NaoH(aq) NaCl(aq) + H2O(l)
Moles of NaOH= mass of HCl
= 46 x 11 = 0.506 moles
1000 - Determine the mass of hydrogen Chloride that dissolved to make 250cm3 of solution. (Cl=35.5, H=1.0) (2mks)
Moles of HCl in 250cm3 = 0.506x10 = 5.06
R.M.M of HCl= 1 x 35.5 = 36.5
Mass of HCl= moles x R.M.M
= 5.06 x 36.5
= 184.69g
- Calculate the number of moles of hydrochloric acid in 25cm3 of solution. (2mks)
-
- In an experiment, a piece of Magnesium ribbon was cleaned with steel wool. 2.4g of the clean Magnesium ribbon was placed in a crucible and completely burnt in Oxygen. After cooling, the product weighed 4.0g.
- Explain why it was necessary to clean the Magnesium ribbon. (1mk)
- To remove the oxide layer which interfere the reaction of magnesium with oxygen gas.
- What observation was made in the crucible after burning? (1mk)
- White solid of magnesium oxide observed.
- Why was there an increase in mass? (1mk)
- Due to addition of oxygen to magnesium ribbon to form magnesium Oxide.
- Write the equation for the reaction which took place in the crucible (1mk)
- 2Mg + O2 → 2MgO
- The product in the crucible was shaken with water and filtered. Explain the observation which was made when blue and red litmus papers were dropped into the filtrate. (2mks)
- Blue litmus paper remain blue
- red litmus paper turns blue
- Calculate the volume of oxygen gas used during the burning. (O=16, Mg= 24, Molar volume of the gas = 24000cm3 at room temperature) (3mks)
2Mg + O2 2MgO
2.4g
Moles = Mass 2.4g = 0.1 moles
R.A.M 0.4 = 0.1 moles
2 → 0.1 moles
1 → x
(1 x 0.1) = 0.005 moles
2
Moles of O2 = 0.005 moles
1 mole 24,000cm3
0.005 x
0.005x 24000 =120cm3
1
- Explain why it was necessary to clean the Magnesium ribbon. (1mk)
- The flow chart below shows the large scale manufacture of Sulphuric acid.
- Why is the purifier necessary? (1mk)
- Purifier removes impurities which would reduce the surface area of the catalyst making it less efficient.
- Name the catalyst used in contact process. (2mks)
- Catalyst is Vanadium (V) Oxide
- Name and give the formula of substance P (1mk)
- P - is Oleum (H2S2O7
- Write the equation for the formation of substance P. (1mk)
SO 3(g) + H2SO4(l) H2S2O7 - State the optimum temperature and pressure for the contact process.
- Pressure. (1mk)
- Optimum pressure 2-3 atmosphere
- Temperature (1mk)
- Optimum temperature 450°C
- Pressure. (1mk)
- Sulphur (IV) Oxide emitted in this process undergoes process known as scrubbing. What is scrubbing?
Write an equation that results to calcium sulphate and water. (3mks)- Neutralizing Sulphur (IV) oxide by calcium hydroxide
Ca(OH)2(s) + SO2(g) CaSO3(aq) + H2O (l)
- Neutralizing Sulphur (IV) oxide by calcium hydroxide
- When compound P is heated with concentrated Sulphuric acid, a gas which forms dense white fumes with ammonia is liberated.
- Identify the anion in P. (1mk)
- Chloride Ion (Cl-)
- Write an ionic equation for the reaction between a solution of P and silver nitrate solution. (1mk)
- Ag+(ag) + Cl- → AgCl(s)
- State what would be observed if lead (II) Nitrate solution was added to a solution of P and mixture heated. (2mks)
- A white precipitate of lead (II) Chloride is formed when heated the precipitate dissolves to form a colourless solution.
- Identify the anion in P. (1mk)
- Why is the purifier necessary? (1mk)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Form 3 Term 3 Opener Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students