Questions
INSTRUCTIONS:
- Answer all the questions in the spaces provided.
- Below is a table of oxides of period 3 elements. Use it to answer the questions that follow.
Element Na Mg Al Si P S Cl Oxides Na2O
Na2O2MgO Al2O3 SiO2 P2O3
P2O5SO2
SO3Cl2O7 Nature of oxides I II III IV V VI VII - Compare the electrical conductivity of oxides of Sodium to those of phosphorus. (2 mks)
- Which oxides would react with dilute Sulphuric acid. Explain. (2 mks)
- Write down balanced equation to show how one of the oxides would react with both 2M hydrochloric acid and 2M Sodium hydroxide solution. (2 mks)
- What structure would be formed by the oxides of silicon? Explain your answer. (2 mks)
The melting point of SO2 is lower than that of MgO. MgO has a giant ionic structure while SO2 has a simple molecular structure. - Describe how you would prepare a dry sample of lead (II) nitrate from the following reagents. Lead (II) oxide and dilute nitric (V) acid. (3 mks)
- Alum used in treating water is both advantageous and disadvantageous. Explain.(2 mks)
- When a blue litmus paper is dipped in a solution of aluminium Chloride it turns red. Explain.
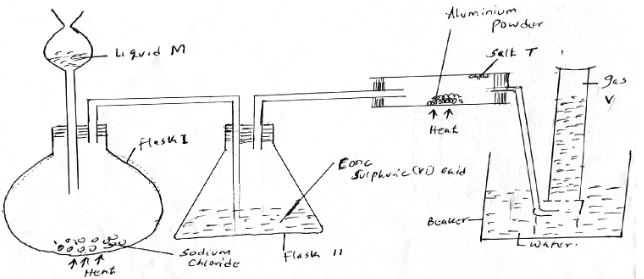
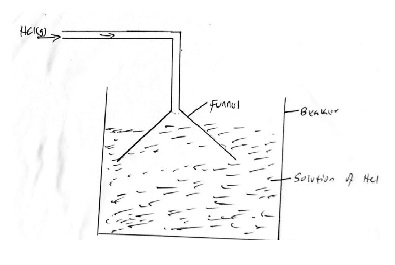
- The set up below was used to prepare hydrogen chloride gas and salt T. (1 ½ mks)
- Identify the following:
- Liquid M.
- Gas V
- Salt T
- Write down balanced chemical equations for reactions that occur at: (2 mks)
- Flask I
- Combustion tube.
- Name the process that formed salt T as shown in the diagram. (½ mks)
- Sulphuric (VI) acid is used as a drying agent in this experiment. Explain why calcium oxide is unsuitable for the same purpose in this reaction. (1 mk)
- The water in the beaker was found to have a pH of 2.0 at the end of the experiment. Explain. (1 mk)
- Calculate the mass of salt T formed if 480cm3 of hydrogen chloride gas measured at room temperature was reacted with aluminium powder (Al = 27, Cl = 35.5 MGV = 24dm3)(2 mks)
- Draw a well labeled diagram showing how you would dissolve hydrogen chloride in water. (2 mks)
- Explain why hydrogen chloride gas dissolved in methyl benzene does not react with calcium carbonate. (1 mk)
- Using an equation, state the observation made when a gas jar containing hydrogen chloride gas is opened near an open bottle of liquid ammonia. (1 mk)
- Identify the following:
- Use the information below to answer the questions that follow.
Ca(s) + ½ O2(g) → CaO(s) ∆H = - 635kj/mol-1
C(s) + O2(g) → CO2(g) ∆H = - 394kj/mol
Ca + C + 3/2O2 → CaCO3(s) ∆H = - 1207 kj/mol- Calculate the enthalpy change for the reaction. (3 mks)
CaO(s) + CO2(g) → CaCO3(s) - State one factor that should be considered when choosing fuel for cooking. (1 mk)
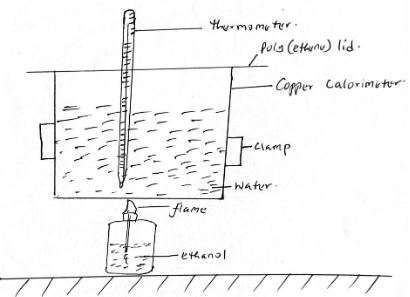
- A student used the apparatus shown to calculate the energy released when ethanol burns. The energy released by the burning ethanol raises the temperature of the water in the copper colorimeter.
Volume of water used = 500cm3
Initial temperature of water = 25oC
Final temperature of water = 44.5oC
Mass of ethanol + Lamp before burning = 121.5g
Mass of ethanol + lamp after burning = 120.0g
Calculate the:- Heat evolved during the experiment (density of water = 1g/cm3, specific heat capacity of water = 4.2jg-1K-1) (3 mks)
- Molar heat of combustion of ethanol. (3 mks)
(C = 12, O = 16, H = 1)
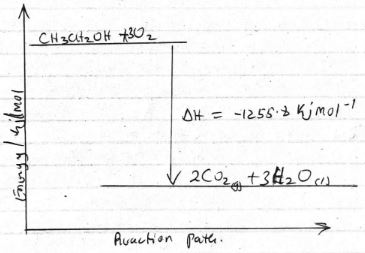
- Write the thermo equation for the complete combustion of ethanol. (1 mk)
- Sketch a simple energy diagram for the reaction of ethanol in air. (3 mks)
- Calculate the enthalpy change for the reaction. (3 mks)
- Name the following compounds using the LUPAC system.
-
- CH3CH2COOCH2CH3 (1 mk)
- CH3CH2CHC=CH (1 mk)
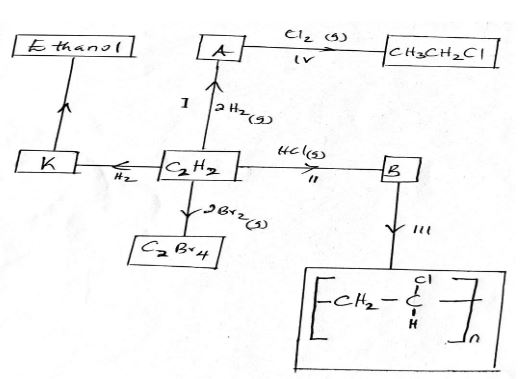
- Study the scheme below and answer the questions that follow.
- Identify the catalyst used in step I. (1 mk)
- Name the compounds A and B. (1 mk)
A
B - Give one disadvantage of compound formed in step III. (1 mk)
- Name the reactions taking place at steps. (2 mks)
III
IV
- Compound A and B have the same molecular formula C3H6O2. Compound A liberates Carbon (iv) oxide on addition of aqueous Sodium carbonate while compound B does not. Compound B has a sweet smell. Draw the possible structures of:
- Compound A (1 mk)
- Compound B. (1 mk)
- A student mixed equal volumes of ethanol and butanoic acid. He added a few drops of concentrated Sulphuric (vi) acid and warmed the mixture.
- Name and write the formula of the main products. (2 mks)
- Which homologous series does the product named in (i) above belong? (1 mk)
-
- Use the standard electrode potentials given below to answer the questions that follow.
Element Volts(V)
Ag+(aq) + e- → Ag(s) + 0.80
Cu2+(aq) + 2e- → Cu(s) + 0.34
Pb2+(aq) + 2e → Pb(s) - 0.13
Zn2+ (s) + 2e- → Zn(s) - 0.76- Select two half-cells which when combined give the lowest workable cell (lowest e.m.f). (1 mk)
- Calculate the e.m.f of the cell formed by combining the two half-cells in (a) above.(1 mk)
-
- Select the strongest oxidizing agent. (½ mk)
- Strongest reducing agent (½ mk)
- A cell was set up using lead and zinc electrodes as shown below.
- Write down the half equation for the half-cell in which oxidation occurs. (1 mk)
- Write down the overall cell equation. (1 mk)
- What is the role of the salt bridge (2 mks)
- An iron cup was electroplated using chromium. The chromium electrode and the iron cup was thoroughly cleaned and weighed before being dipped into the electrolyte.
- Why was it necessary to clean the metals before dipping them into the electrolyte? (1 mk)
- A current of 0.75A was passed through the solution for one hour and four minutes. The mass of chromium deposited on the cup was 0.52g (1 Faraday = 96500C, Cr = 52)
- Calculate the quantity of electricity. (1 mk)
- How many moles of chromium were deposited? (1 mk)
- Calculate the quantity of electricity to deposit one mole of chromium.(2 mks)
- Calculate the number of Faradays required to deposit one mole of chromium and hence deduce the change of ion. (2 mks)
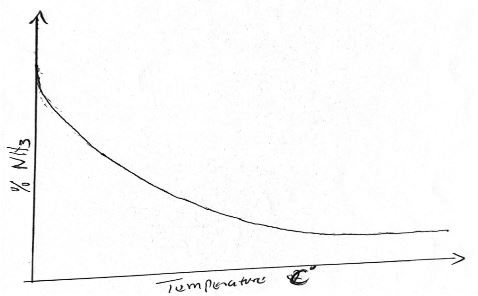
- Nitrogen and hydrogen react to form ammonia gas as shown in the following equation.
N2(g) + 3H2(g) 2NH3(g) ∆H = -97 Kj/mol
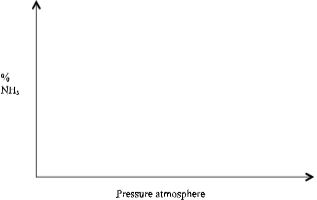
The figure below shows how the percentage of ammonia gas in the equilibrium mixture changes with temperature- Explain why the percentage of ammonia gas changes as shown in the figure above. (2 mks)
- On the following axis, sketch a graph showing how the percentage of ammonia gas in the equilibrium mixture changes with pressure. (1 mk)
- Bismuth chloride (BICl3) reacts with water according to the equation given below.
BiCl3(g) + H2O(l) → BiOCl(s) + 2HCl(aq)- State what would happen when a few drops of dilute hydrochloric acid are added to the mixture at equilibrium. (1 mk)
- Give a reason for your answer in (a) (i) above. (1 mk)
-
- In that harbor process, the industrial manufacture of ammonia is given by the following equation:
N2(g) + 3H2 → 2NH3(g) ∆H = -97jh/mol- Name one source of hydrogen used in the process. (1 mk)
- Name the catalyst used in the above reaction. (1 mk)
- What is the effect of increasing temperature on yield of ammonia. Explain. (2 mks)
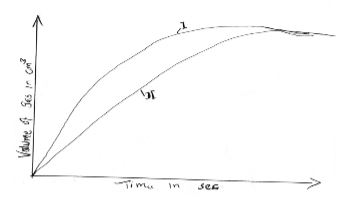
- The curves below were obtained when equal volumes of 2M HCl were reacted with 3.0g of marble chips (CaCO3). In one of the reactions, the acid was warmed before adding this marble chips.
- Write down the equation for the reaction. (2 mks)
- Identify the curve representing the reaction where the acid was warmed. (1 mk)
- In that harbor process, the industrial manufacture of ammonia is given by the following equation:
Marking Scheme
-
-
- Oxide of sodium are good conductors in aqueous solution and molten form due to the presence of mobile ions
- Oxides of phosphorus are non conductors in all states since they lack mobile ions.
- Oxides of sodium, magnesium and aluminium. These are basic oxides.
- Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(l)
Al2O3 (s) + 2NaOH(aq) → 2Na(Al(OH)4)(aq) + 3H2O(l) - Giant atomic or giant covalent structure, silicon and oxyegn have small differences in electron affinity.
Continuous covalent bonds exists in the structure of the oxides to create stable compounds - Add dilute nitric (V) acid to lead (II) oxide and stir until all the oxide has dissolved.
Filter the mixture to obtain a nitrate
Evaporate the filtrate until the crystals begin to appear on a string rod.
Allow to cool for crystals to form.
Drain off the excess filtrate and dry in the sun. - Alum causes acidity therefore disadvantageous
Alum coagulates dissolved or suspended particles therefore purifier - Aluminium chloride hydrolises in solution producign hydroxium ions which turn blue litmus paper red.
-
-
-
- Liquid M - concentrated sulphuric (VI)acid
- Gas V - Hydrogen
- Salt T - Aluminium(III)chloride
-
- Flask I
NaCl(s) + H2SO4(l) → NaHSO4(aq) + HCl(aq) - Combustion tube
2Al(s) + 6HCl(g) → 2AlCl3(s) + H2(g)
- Flask I
- Sublimation
- It results with hydrogen chloride gas to form a salt.
- Excess HCl dissolved in the water to form hydrochloric acid.
- MGV = 24dm3
MOles of HCl
= 480/24000 = 0.002 moles
Moles of AlCl3 =
0.02 x 2 = 0.00667
6
Mass = 0.00667 x 133.5
0.89g -
- HCl in methylbenzene exists as a molecule: no free ions
- HCl(g) + NH3(g) → NH4Cl(s)
(white fumes)
-
-
-
ΔH= ΔHcCa + ΔHcC = ΔH CaCO3
-635 + - 394 = -1207
ΔH = -1207 + 635 + 394
= -178KJmol-1 -
- Heating value
- Availability
- Cost
- Ease of storage
- Ease of combustion
- Efficiency of environment.
-
- Mass of water 500cm3 x 1g/cm3 = 500g
ΔT = 44.5 - 25.0 = 19.5C
Heat evolved = 500g x 4.2jg-k- x 19.5
= 409.50j - Mass of ethanol = 121.5 - 120.0 = 1.5g
RMM of ethanol = CH3CH2OH = 46
1.5g of ethanol produced 409.50j
40 → x
(409.50 x 46)= 1255800j/mol
= 125.58KJ
- Mass of water 500cm3 x 1g/cm3 = 500g
- CH3CH2OH + 3O2 → 2CO2 + 3H2O ΔH = -1255.8KJ/mol
-
-
-
-
- Ethylpropanoate
- 3-BromoHex-2-ene
-
- Nickel/ pakadium
- A- Ethane
B- chloroethene - Non- biodegradable
- III- polymerisation
IV - substitution reaction
- Nickel/ pakadium
-
- CH3CH2COOH
- CH3COOCH3 or HCOOCH2CH3
- CH3CH2COOH
-
- CH3CH2CH2COOCH2CH3
Ethylbutanoate - Esters or Alkylalkanoate
- CH3CH2CH2COOCH2CH3
-
-
- Pb2+ + 2e- ⇌ Pb(s)
and
Cu2+ + 2e- ⇌ Cu(s) - Ered - E oxide
0.34 + 0.13 = +0.47V -
- Ag+
- Zn(s)
-
- Zn(s) ⇌ Zn2+ + 2e-
- Zn(s) + Pb2+(aq) ⇌ Zn2+(aq) + Pb(s)
-
- To remove any oxide layer that might interfere with electroplating process.
-
- Q= 1t
= 0.75 x 3840
= 2880C - Moles = mass/RAM
0.54 = 0.01moles
52 - 0.01 moles requires 2880C
1 moles " ' x
1 x 2880C = 288000C
0.01 - 1F → 96500C
xF → 288,000C
x(1F x 288000C)
96500
x = 2.98F
charge = 3+
- Q= 1t
- Pb2+ + 2e- ⇌ Pb(s)
-
- As temperature increases, the yiled of ammonia gas dereases since forward recatio, exothermic.
-
- equilibirum shifts to the left.
- In order to reduce the concentration of HCl and restore the equilibrium.
-
-
- Cracking of long chain alkanes
- Finely divided iron/ iron powder.
- Yield decreases
High temperature favours background reaction.
-
- CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
- Cave I
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Form 4 End Term 1 Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students