INSTRUCTIONS TO CANDIDATES
- Answer all the questions in the spaces provided after each
- All working must be clearly shown where necessary.
- All answers should be written in English.
-
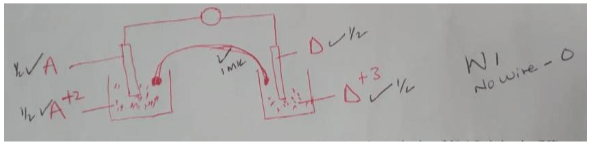
- The following table gives the standard electrode potentials for some half cell reactions
Half cell reations Eθ (v)- A2+(aq) + 2e− → A(s) −0.76
- B2+(aq) + 2e− → B(s) −0.44
- C2(aq) + 2e− → 2C−(aq) +0.54
- D3+(aq) + e− → D2+(aq) −0.77
- 2E+(aq) + 2e− → E2(g) 0.00
- Identify the strongest reducing agent. (1mk)
- Calculate the EMF of a cell made by combining the half cells of A and B. (1mk)
- Identify the substances that can oxidize C- ions to C2. (1mark)
- Draw an electrochemical cell comprising half cells I and IV. (3marks)
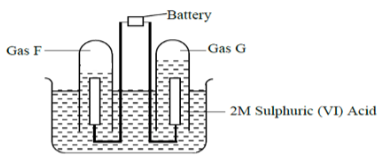
- The apparatus below shows the set up that was used in the electrolysis of 2M Sulphuric (VI) acid. Study it and answer the questions that follow
- Write an equation for the reaction that produce gas F. (1 mark)
- Describe how gas G can be identified (1 mark)
- 1.9g of a metal F was deposited when its aqueous salt was electrolysed by passing a current of 0.6A for 1.5hours. Determine the charge on the ion of F.(RAM of F = 113, 1F = 96500C) (3mks)
- The following table gives the standard electrode potentials for some half cell reactions
- The diagram below shows a blast furnace which is used for the extraction of iron. Study it and answer the questions that follow.
- Name two major ores from which iron can be extracted from (1mk)
- Give a reason why the temperature at the bottom of the furnace is very high (1mark)
- State two properties of slag that allows it to be separated from molten iron. (2mark)
- Write an equation for the reaction responsible for the fall in temperature at stage 2. (1mark)
- Write an equation in which iron is formed. (1mk)
- State two uses of iron (1mark)
- State the effect of iron extraction on the environment (1mark)
- Sulphuric (VI)acid is manufactured by the contact process .The equation below shows one step involved in the contact
2SO2(g) + O2(g) ⇌ 2 SO3 (g) ∆H= −97kJ/mol- State giving reasons how an increase in temperature would affect the amount of sulphur VI) oxide gas. (2MK)
- Name the catalyst used in the above process (1mark)
- State any two uses of sulphuric(VI) acid (1mark)
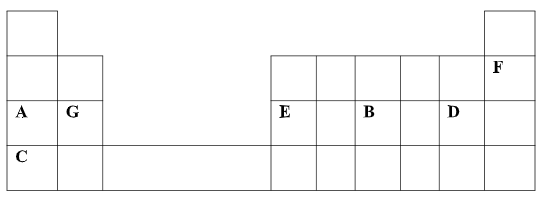
- The table below shows some elements in the periodic table. Use it to answer the questions that follow. The letters are not the actual symbols of the elements.
-
- Show the electron arrangement of elements:
- A (½ mk)
- D (½ mk)
- Using dots (.) and crosses (X) to represent electrons, draw diagrams to show how elements C and oxygen combine to form a compound.( O= 8) (1mk)
- Show the electron arrangement of elements:
- Show on the grid above an element Y belonging to Period 4 and group (VI). (1mk)
- Compare the following with explanations:
- The reactivity of A and C (2mks)
- Atomic radii of elements A and B (2mks)
- The melting point point of the oxide of element G and and the oxide of D (2MKS)
- Calculate the volume of the gas produced when 0.92g of element A is reacted with excess dilute hydrochloric acid at room temperature and pressure. (MGV= 24dm3 , A= 23). (3MKS)
-
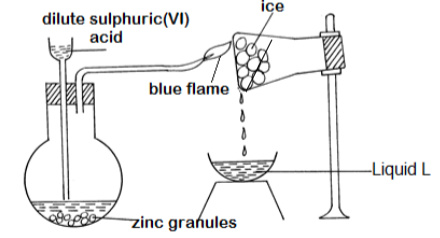
- The diagram below shows a set-up of apparatus that was used to prepare hydrogen gas.
- Explain the observations that would be made if calcium turnings were used instead of zinc granules in the above experiment. (2mks_
-
- Explain how liquid L can be identified by chemical means. (2mks)
- How could the purity of liquid L be confirmed?(1mk)
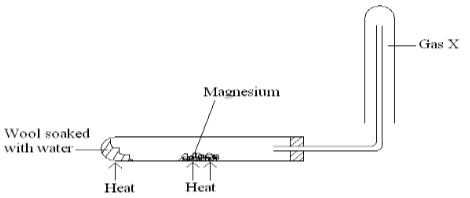
- When magnesium is heated in steam it reacts rapidly forming a white solid and gas X.
- Write an equation that took place in the heated test tube. (1mk)
- Why is the gas X collected as shown in the diagram above? (1mk)
- Study the flow diagram below and answer the questions that follow:
- Write a balanced equation for the reaction between hydrochloric acid and manganese (IV) oxide that produces gas L (1mk)
- State and Explain what happens to a blue litmus when dropped into a solution of gas L (3mk)
- Name the salt that will be formed if the experiment was repeated with hot concentrated NaOH. (1mk)
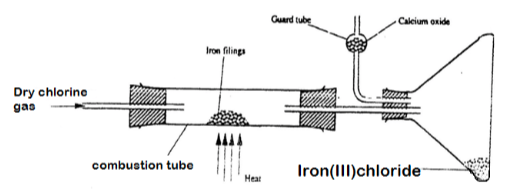
- A student carried out an experiment to prepare iron III chloride using the apparatus shown in the diagram below
- Explain why:
- It is necessary to pass chlorine gas through the apparatus before heating begins.(1mk)
- Calcium oxide would be preferred to calcium chloride in the guard tube. (1mk)
- The iron iii chloride is collected away from the reaction point. (1mk)
- Calculate the mass of the product that would be formed when 4000cm3 of chlorine gas reacts completely with excess iron fillings ( Fe= 56.0, C1 = 35.5, MGV 24 litres at room temperature and pressure.). (2MKS)
- Explain why:
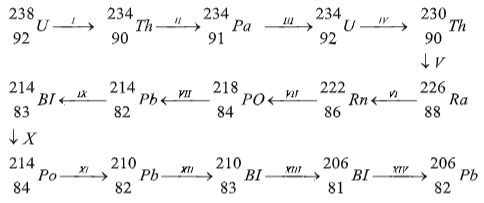
- Study the decay series below and answer the questions that follow.
- Define the term radioactivity.(1mk)
-
- Identify the particles emitted insteps (I) (1mk)
- Steps (I) (1 mk)
- Steps (IX) (1mark)
- Write the nuclear equation for the reactions which take place in step(V) (1mark)
- Calculate the mass of a radioactive element W that would remain after 30 days if it has a half life of 6 days and the original mass is 48gramms. (2mks)
- Identify the particles emitted insteps (I) (1mk)
- Explain any two applications of radioactivity in medicine.(2mks)
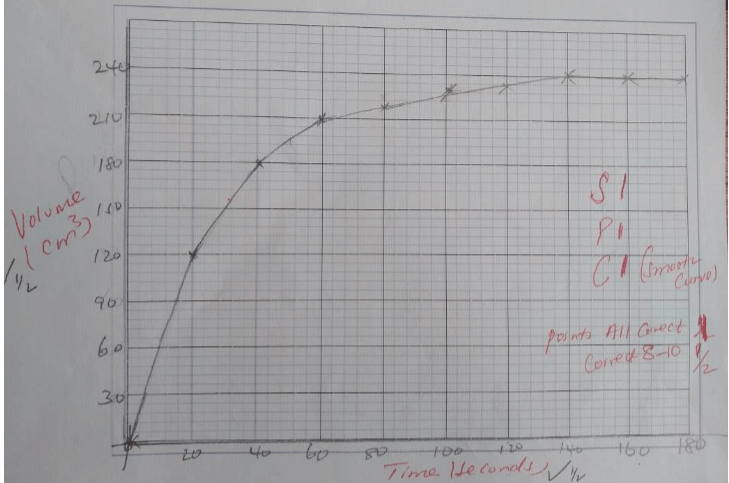
- In an experiment to study the rate of the reaction ,1gram of lamps of calcium carbonate was added to 300cm3 of 2M hydrochloric acid at 250 c. The volume of the carbon( iv) oxide produced was measured at 10 second intervals . The results obtained were recorded in the table below.
TIME (SECONDS) 0 20 40 60 80 100 120 140 160 180 VOLUME (CM3) 0 120 180 210 224 232 236 240 240 240 - Plot a graph of volume of the gas produced against time . (3mks)
- Use the graph to find the ;
- Volume of gas produced after 50 seconds. (1mk)
- Time needed to produce 155 cm3 of carbon (iv) oxide. (1mk)
- EXPLAIN why the volume of carbon (iv) oxide produced does not exceed 240cm3 (2mk)
- Calculate rate of the reaction at the 55th minute. ( 3mks)
- LIST any two ways in which the reaction above can be fastened.(2mk)
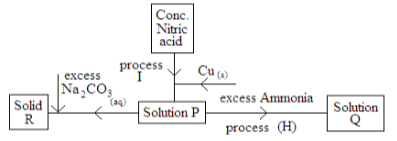
- Study flow chat below and answer the questions that follow.
- Write a chemical equation to show how solid R is formed. (1mk)
- State the observation that would be made in process H if ammonia solution was replaced with excess sodium hydroxide solution.(1mk)
- State any two observations made in process I (2mk)
- Write the two ionic equations that led to the formation of solution Q (2MK)
- State any two uses nitric (v) acid (2mk)
MARKING SCHEME
-
-
- A
- −0.44− −0.76{ ½ )
=0.32V { ½ ) - A B,E NB/ all 1mk, any 2 ½
-
-
- 2H(aq)++2eH2(g)
- Introduce a glowing splint and it will relight.
- Mass deposited=QRAM/FC
Let charge be C
1.9=[0.6x1.5x60x60] x113/[96500×C]
C=[0.6x1.5x60x60x113]/[1.9x96500]
C=1.9968 or ~ +2
OR
Q= IT
0.6X1.5X60X60=3240
If 3240=1.9g
? =113
[3240X113] =192694.73c
1.9
192694 =1.9968
96500
~+2
-
-
-
- Haematite
- Magnetite
- reaction of coke with oxygen is highly exothermic
-
- Low density.
- Immiscible with iron.
- CO2 + C ……… 2CO
- 2Fe2O3 + 3C ……….4Fe + 3CO2
-
- cast iron is used to make manhole covers
- used as a catalyst in harber process
- making cutlery and surgical equipments
-
- SO2 causes acidic rain
- CO2 Causes global warming
-
- It would decrease{1mks},increase in temperature favours endothermic reactions ½ and therefore reverse reaction is favoured ½
- anadium (iv) oxide ~reject platinum.
-
- Manufacture of dyes{1/2mks}
- Filling batteries{1/2mks}
Any other correct answer
-
-
-
-
- A 2,8,1
- D 2,8,7
-
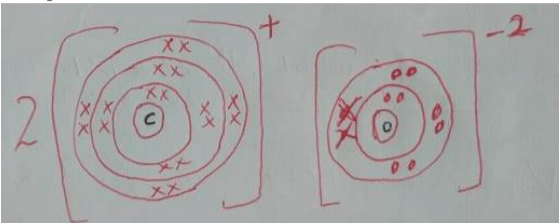
-
- Yshown in the table,period4 group vi
-
-
- C is more reactive than A{1mks}
- C has a larger atomic radius/lower ionization energy and therefore easily loose the outer most electron{1mks}
- A has a larger atomic radius than B /B has a shorter atomic radius than A[{1mks}B has more protons than A and therefore energy level attracted strongly towards the nucleus.{1mks}
- Oxide of G has the higher melting point than the oxide of D.{1mks} Because oxide of G has a giant ionic structure with strong ionic bond while oxide of D has simple molecular structure with weak van der waals forces.{1mks}
-
- 2Na +2HCl 2NaCl +H2 1mk
Moles of A = 0.92 ( ½ )
23
= 0.04moles
Mole ratio
2 : 1
Moles of hydrogen = 0.04
2
= 0.02 ( ½ )
Volume of hydrogen
0.02 X24 = 0.48dm3 or 480cm3
-
-
- The reaction would start and then stop immediately {1mks}
Because calcium sulphate formed is insoluble and therefore forms a coating on calcium preventing further reaction ( 1mk) -
- Add a sample to;
White anhydrous copper (ii) sulphate, it would turn to blue.
OR
Blue anhydrous cobalt (ii) chloride, it would to pink.
NB/ REAGENT 1MK, CORRECT COLOUR CHANGES 1MK - Boil ½ , If it boils at a constant/fixed temperature. ½ REJ 1000 C
- Add a sample to;
-
- Mg(s) + H2O(g) MgO(s) + H2(g)
- It is less dense than air.
- The reaction would start and then stop immediately {1mks}
-
- MnO2(s) +4HCl(aq) MnCl2(aq) + Cl(g) +2H2O(l)
-
- It turns red then white{1mks}
- Red because the solution is acidic{1mks}
- White because the HOCl bleaches the dye in the litmus{1mks}
- sodium chlorate
-
-
- To expel the air that was inside so that its oxygen doesn’t react with chlorine.
- It would absorb both water moisture and un-reacted chlorine.
- moles of chlorine gas = 4000.
24000
= 0.16677moles
2Fe + 3Cl2 → 2FeCl3 {1mks}
Mole ratio
Cl2 : FeCl3
3 : 2
Moles of product
If 3 → 0.1667
2?
2 × 0.1667
3
= 0.11111moles
Mass of product = moles × RMM
0.11111 × 162.5
=18.0538g
-
-
- It is the spontaneous disentigration of an unstable nuclide to form a stable nuclide.
-
-
- Step (I) Alpha
- Step (ix) Beta β
- 90230Th 88226Ra + 24He
- 30/6 = 5
48 → 24 → 12 ----- 6 → 3 → 1.5g
=1.5g
-
-
- Radioactive iodine is used in imaging the thyroid gland.
- For therapy, radioactive materials are used to kill cancerous tissue, shrink a tumor or reduce pain.
- Nuclear medicine uses radiation to provide diagnostic information about the functioning of a person's specific organs, or to treat them
-
-
-
- 240 - value at 50 {1mks} Answer {1mks}
- value to be read from the gragh.
- Because 1g of calcium carbonate can only produce 240cm3(1mk)
That is 1/100 = 0.01 (½ )
Moles of CO2
CaCO3 ;CO2
1 : 1
0.01×24000
=2 40cm3 - Tangent at 55th minute (1MK)
Dy/dx {1mks}- Answer with units {1mks}
- Answer with no units{1/2mks}
-
- Use powdered calcium carbonate.
- Warm the acid.
-
-
- Cu(NO3)2(aq) + Na2CO3(aq) CuCO3(S) +2NaNO3(aq).{1mks}
- A blue precipitate, ½ Insoluble in excess.{ ½ mks}
- Brown copper solid dissolves forming a blue solution.
Brown fumes are seen escaping. - Cu(aq)+2 +2OH(aq) → Cu(OH)2
Cu(OH)2(S) + 4NH3(aq) → [Cu(NH3)4](aq)+2 +2OH-(aq) -
- To clean metals.
- Manufacture of nitrogeneous fertilizers
Any other relevant
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Form 4 End Term 2 Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students