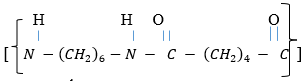INSTRUCTIONS TO CANDIDATES: -
- Answer all the questions
- Candidates should answer the questions in English.
- The table below represents some elements. Use it to answer the questions that follow. The letters do not represent the actual symbols of the element.
Element Atomic Number Melting point °C Q
R
S
T
U11
13
14
17
1997.8
660
1410
−101
63.7- Write the electron arrangement for the ions formed by elements.
- R ( 1 mk)
- T ( 1 mk)
- Select an element which is ;
- The most reactive metal ( ½ mk)
- A semi-conductor of electricity. ( ½ mk)
- Compare the atomic radius of Q and R. Explain ( 2mk)
- Use dots (.) and crosses (x) to represent electrons, show the bonding formed between S and T. (1mk)
- Explain why the melting point of S is higher than that of Q (2mks)
-
- Write an equation for the reaction between Q and water. (1mk)
- Calculate the mass of the solid formed when excess R reacts with 960cm3 of oxygen gas. (MGV= 24dm3, O=16, Al=23) (2mk)
- Write the electron arrangement for the ions formed by elements.
-
- Use the information below to answer questions that follows
Ca(s) + ½O2(g) → CaO(s) ∆H = −635kJ/mole
C(s) + O2(g) → CO2(g) ∆H = −394kJ/mole
Ca(s) + C(s) + 3/2O2(g) → CaCO3(s) ∆H = −720kJ/mole
Calculate the enthalpy change for the reaction
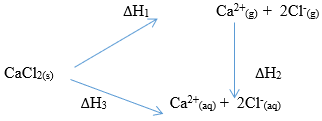
CaO(s) + CO2(g) → CaCO3(s) (3mks) - Study the energy cycle below and use it to answer the questions that follow.
- What name is given to the enthalpy change DH2 (1mk)
- Given that DH1 = +2237kJ/mole and DH2 = −2378kJ/mole. Calculate the value of DH3 (2mks)
-
- When 1.2g of element W was completely burnt in oxygen and all the heat evolved used to heat 250cm3 of water. The temperature of the water rose from 22°C to 29°C. Calculate the RAM of element W. (Specific heat capacity 4.2j/g-1k-1 , density of water 1.0g/cm3 and molar heat of combustion is -560kJ/mole) (2mks)
- STATE two reasons why the molar heat of combustion of the above compound is different from the theoretical value. (1mk)
- Define the term, enthalpy of solution. (1mk)
-
- Use the bond energies given below to calculate the heat of reaction for.
H2(g) + Cl2(g) → 2HCl(g) (2mks)
Bond H - H Cl - Cl H - Cl Bond energy kJ/mole 435 243 431 - Sketch the energy level diagram for the above reaction. (1mk)
- Use the bond energies given below to calculate the heat of reaction for.
- Use the information below to answer questions that follows
-
- Starting with Zinc metal, describe how zinc carbonate can be prepared in the lab. (3mks)
- Three immiscible liquids Y, X and Z are such that X is less dense than Z and Y is denser than Z. Draw a well labeled diagram that can be used to separate the three liquids effectively. (3mks)
- When carbonate of metal T was heated, solid P which turned yellow on cooling was formed.
- State the identity of solid P (1mk)
- Write a balanced chemical equation to show the effect of heat on the nitrate of silver. (1mk)
- Few drops of ammonium hydroxide were added to copper II sulphate solution in a test tube.
- State the observation made (1mk)
- If excess ammonium hydroxide is added to the resultant product above, state the observation made and write the equation for reaction that took place (2mk)
-
- The table below shows volume of hydrogen gas produced when 0.00167g of zinc granules reacted with excess of dilute HCl
Time (mins) 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 Volume (cm3) 0 10 18 24 28 31 34 37 38 39 40 40 40 - Plot a graph of volume of hydrogen gas produced against time. (3mks)
- From the graph find the rate of reaction at minute 3.5 (2mks)
- Explain why the rate of reaction
- Is faster at the beginning of the experiment (1mk)
- Remains constant from the 5th minute (1mk)
- On the same axes sketch a graph for the reaction between the 2g zinc powder and 100cm3 of 2MHCl. (1mk)
- In the production of hydrogen for use in Haber process, carbon (II) oxide is reacted with steam according to the following equations
CO(g) + H2O(g)CO2(g) + H2(g) ∆H=+ve
- What name is given to the above type of reaction (1mk)
- State and explain the effect of
- Removing carbon (IV) oxide from the mixture on the yield of hydrogen. (1 ½ mks)
- Increasing the temperature of the system on the equilibrium mixture. (1 ½ mks)
- The table below shows volume of hydrogen gas produced when 0.00167g of zinc granules reacted with excess of dilute HCl
-
- Define the following term Binary electrolyte (1mk)
- A form four student from MSHINDI secondary school was given the following list of elements with their reduction potentials.
element Electrode potential G 0.0 Z − 0.6 M + 1.82 V − 1.9 Y + 0.9 J − 2.4 - State the possible identity of element G and give a reason for your answer. (1mk)
- Draw a well labelled diagram of the electrochemical cell by combining the half-cells of element Y and J (3mk)
- Calculate the emf of the cell above. (1mk)
- Write the cell representation of a cell made by combining the half-cell of element M and Y.( Take their charges to be +2 respectively.) (1mk)
- In the electrolysis of dilute sulphuric (VI) acid using platinum electrode, the volume of hydrogen collected is twice the volume of oxygen collected. Explain this observation. (2mk)
-
- A current of 1.3 Amperes was passed through an electrolytic cell containing Copper ii sulphate solution for 2½ hours using graphite electrodes. Calculate the mass of copper deposited at cathode. (Faraday= 96500C, Cu= 63.5) (2mk)
- Apart from the deposition of brown copper metal, state another observation seen during the electrolysis.(1mk)
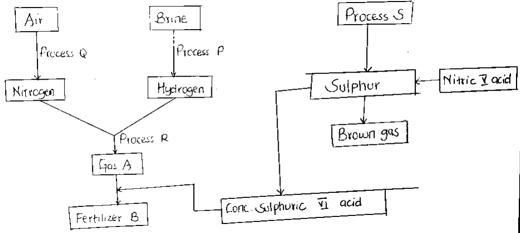
- Study the flow chart below and answer the questions that follows
- Name the following ( 1½ mk)
- Process Q
- Process R
- Process S
- Write equations for the formation Of Fertilizer B (1mk)
- Name the catalyst and give conditions for process R.
- Catalyst ( ½ mk)
- Conditions (1mk)
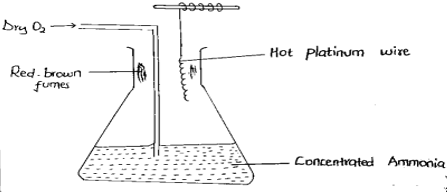
- Study the set up below and answer the questions that follow
- Write an equation for the reaction that takes place. (1mk)
- Explain the source of red brown fumes. (1mk)
- Describe a chemical test that can be used to distinguish between sulphur IV oxide gas and hydrogen sulphide gas. (2mk)
- Name the following ( 1½ mk)
-
- Name the following compounds. (2mks)
- CH3CH2COOH
- CH3CHClCHBrCH2CH3
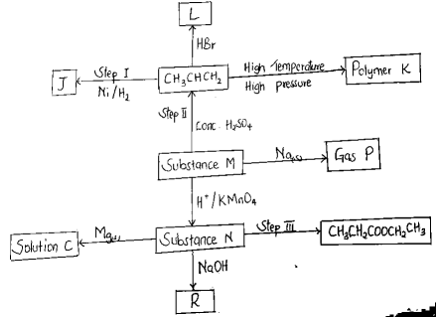
- Use the flow chart to answer the questions that follow
- Name the following (1½ mks)
- Gas P
- Solution C
- Substance J
- Name the type of reaction involved in the following steps. (1½ mks)
- Step I
- Step II
- Step III
- Draw and name the structure of Polymer K (2mk)
- Write a chemical equation for the reaction taking place during the formation of substance L. (1MK)
- Name the following (1½ mks)
-
- Study the structure below
- Name the structure above. (1mk)
- Draw the structures of the two monomers that make up the above structure. (2mks)
HN-(CH2)6-N-C-(CH2)4-C
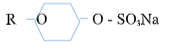
- Below are two cleansing agents. Study them and answer the questions that follow
B- R – COONa
C -- Name the class to which cleansing agent B belongs? (1mks)
- Which cleaning agent above is not environmentally friendly? Explain (2mks)
- Study the structure below
- Name the following compounds. (2mks)
MARKING SCHEME
-
-
- 2,8
- 2,8,8
-
- U
- S
-
- Q has a larger atomic radius than R/ R has a shorter atomic radius Q 1mk
- R has a greater nucleic charge /protonic charge which attracts the energy levels reducing its size
- S has a giant atomic structure with strong covalent bonds while T HAS SIMPLE MOLECULAR STRUCTURE WITH WEAK VAN DER WAAL FORCES.
-
- 2Na + 2H2O -------------- 2 NaOH + H2
2Q + 2H2O ---------------2 QOH + H2 - 4Al + 3O2 --------------- 2 Al2O3 1mk
MOLES OF OXYGEN GAS 960 = 0.04 ½mk
24000
MOLES OF AL2O3 O2;AL2O3
3 ; 2
0.04; ?
0.04 X 2 /3 = 0. 0267 ½ mk
Mass of AL2O3 = 0.0267 X 102= ½mk
2.7234g ½ mk
- 2Na + 2H2O -------------- 2 NaOH + H2
-
-
- CaO………………..Ca+ ½O2 +635
CO2………………….C + O2 +394
Ca + 1½O2 …………CaCO3 −720
= +309 -
- DH Hydration
- DH3 = DH1 + DH2
+2237 − 2378 = −141
-
- MCDT M= 250, C= 4.2 ,DT= 29 − 22 = 7
250 X 4.2 X 7 = 7.35kJ
1000
If 1.2g= 7.35
?= 560
1.2 x 560 = 91
7.35 -
- Incomplete combustion of the compound.
- Heat loss to the surrounding
- The enthalpy change when one mole of a substance is dissolved in water to form the most dilution solution possible/an infinitely dilute solution.
- MCDT M= 250, C= 4.2 ,DT= 29 − 22 = 7
-
- H-H + Cl –Cl ---------2H-Cl
+435 + 243 --------------2( -431) 1MK
678 – 862= -184 Kj 1mk - exothermic , reactants above, products below, arrow facing down.
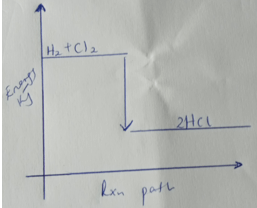
- H-H + Cl –Cl ---------2H-Cl
- CaO………………..Ca+ ½O2 +635
-
-
- React zinc metal with dilute nitric v acid, stir until effervescence stops.
- Filter. React the zinc nitrate solution formed with sodium carbonate to form insoluble zinc carbonate. Filter, rinse the residue with distilled water and dry between filter papers.
- Nb/ separating funnel - 1MK
correct order of liquids- X, Z, Y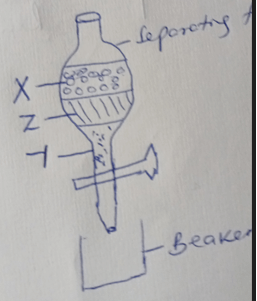
Beaker---------------- ½
Stand ---------------- ½ -
- Lead oxide
- 2AgNO3(aq)………………2Ag(aq) + 2NO2(g) +O2(g )
-
- A blue precipitate is formed. 1mk
- Blue ppt dissolves forming a deep blue solution. 1mk
Cu (OH)2(s) +4NH3(aq) …………..[Cu(NH3)4]+2 (aq) +2OH− (aq)
-
-
-
- x-axis with correct units½
Y-axis with correct units ½
Plotting- 13 points- 1mk
12 points – ½ mk
11points and below- 0mk - tangent- 1mk dy/dx= ½ correct answer with units ½
-
- the reactants are at a higher concentration in the beginning thus more particles hence higher chances of collision 1mk
- all the zinc has been used up and therefore reaction has come to an end.1mk
- curve should start and level off at the same time with the other one but is ABOVE IT.
- x-axis with correct units½
-
- Reversible reaction
-
- The yield would increase.1MK Removing co2 would favor the forward reaction forming more hydrogen.
- Equilibrium shifts to the right. 1mk The forward reaction is endothermic and therefore would be favored by an increase in temperature
-
-
- an electrolyte that contains only one cation and one anion.
-
- Hydrogen. ½ It has an electrode potential of 0.0v ½ mk
-
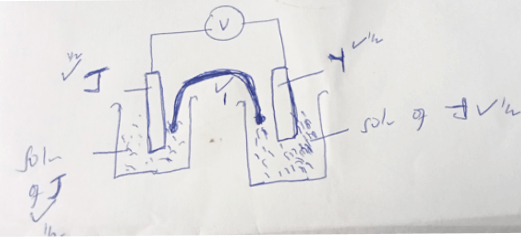
- EREDUCED - EOXIDIZED
+ .9 − −2.4 = ½
0.9 +2.4= 3.3V - Y/Y+2 (aq) // M+2 (aq)/M(S)
- The electrons lost at anode must be the ones gained at cathode 1 mk , 4 electrons are lost at anode to form 1 mole of oxygen, when the four electrons are gained at cathode, 2 moles of hydrogen are formed1mk
also accept correct equations;
4OH− (aq) ------------ 2H2O + O2 + 4e−
4H+ (aq) + 4e− ---------------- 2H2 (g)
Both equations must be correct to score two mks -
- Q=IT 1.3X 2.5 X60 X 60= 11,700C 1mk
63.5g of Cu= 2Faradays
If 63.5g ---------- 2 x96500
? ------------ 11700
63.5 x 11700 ½ mk= 3.8494g ½ mk
2x 96500 -
- blue color of solution fades.
- bubbles of a colorless gas
- Q=IT 1.3X 2.5 X60 X 60= 11,700C 1mk
-
-
- Electrolysis
- Haber
- Frasch
- 2NH3aq + H2SO4aq → ( NH4)2SO4 aq
-
- finely divided iron ½ mk
-
- Temp --450°c ½ mk
- Pressure 200- 250 atm ½ mk
-
- 4NH3 + 5O2----------- 4NO + 6H20
- The NO produced is readily oxidized to NO2
-
- Bubble a sample of each gas into acidified potassium manganite (vii), 1mk
- In both purple acidified KMnO4 will turn colorless but with H2S there will be a yellow deposit. 1mk
-
-
-
- Propanoic acid
- 3-Bromo-2-Chloro-pentane
-
-
- Hydrogen
- Magnesium propanoate
- Propane
-
- I -hydrogenation
- II- dehydration
- III – esterification
- polypropene.
- CH3CHCH2 + HBr--------- CH3CH2CHBr
-
-
- nylon 6,6
- NH2 (CH2)6NH2 1MK and HOOC (CH2)4COOH 1MK
-
- Soap detergents
- C 1mk, it is non-biodegradable 1mk
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - Form 4 Term 3 Opener Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students