Instructions
- Write your name, Index number and class in the spaces provided above.
- Answer ALL the questions in the spaces provided.
- Mathematical tables and silent electronic calculators may be used.
- All working MUST be clearly shown where necessary.
QUESTIONS
-
- Consider the following reaction:
A2(g) + B2(g) →2AB(g), ΔH = +75 kJ
Sketch an energy level diagram showing the relative activation energies for the catalysed and uncatalysed reactions using the axes below. (2mks) - Given that; ΔHf (Al2O3) = – 1590 kJmol-1
ΔHf (Cr2O3) = -1134kJmol-1
Calculate the heat of reaction for; (2mks) - The following data was obtained during an experiment
Mass of ethanol burnt = 0.2g
Mass of water in the calorimeter = 200g
Specific heat capacity of water = 4.2 jg-1k-1
Initial temperature of water = 23.5ºC
Final temperature of water = 28.0ºC- How was the mass of ethanol that burnt determined? (1mk)
…………………………………………………………………………………….…….
…………………………………………………………………………………….……. - How much heat was required to raise the temperature of water from 23.5ºC to 28.0ºC? (2mks)
…………………………………………………………………………………….…….
…………………………………………………………………………………….……. - Two assumptions were made in calculating the enthalpy of combustion for ethanol. State them. (1mk)
…………………………………………………………………………………….…….
…………………………………………………………………………………….……. - Determine the molar enthalpy of combustion of ethanol.(C= 12,H=1, O=16) (2mks)
- Write a thermochemical equation for the combustion of ethanol given the accurate value for enthalpy of combustion is – 1368 kJmol-1. (1mk)
…………………………………………………………………………………….…….
- How was the mass of ethanol that burnt determined? (1mk)
- Consider the following reaction:
- Two half cells were connected as shown to form a voltaic cell. The reduction potentials are given.
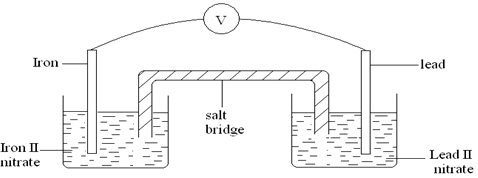
Pb2+(aq) + 2e Pb(s)EӨ = – 0.13V
Fe2+(aq) + 2e Fe(s)EӨ = – 0.44V- Calculate the e.m.f of the cell. (1mk)
- Sodium chloride is used as the salt bridge. State the two functions of the salt bridge. (2mks)
…………………………………………………………………………………….……. - Show the direction of the electron flow in the external circuit. (1mk)
- The e.m.f of the cell will reduce with time. Give a reason for this. (1mk)
…………………………………………………………………………………….……. - During electrolysis of water acidified with Sulphuric acid, two gases were produced at the electrodes:
- State which ions are preferentially discharged at the electrodes. Explain with aid of half ionic equations.
Anode. (2mks)
…………………………………………………………………………………….…….
Cathode. (2mks)
…………………………………………………………………………………….……. - Calculate the volume of the gases at s.t.p produced when a current of 0.025A is passed for 4 hours. (1 Faraday=96500C) (3mks)
- State which ions are preferentially discharged at the electrodes. Explain with aid of half ionic equations.
-
- The fermentation of glucose is catalysed by enzymes from yeast. Yeast is added to aqueous glucose, the solution starts to bubble and becomes cloudy as more yeast cells are formed.
C6H12O6(aq) 2C2H5OH(aq)+2CO2(g)
The reaction is exothermic. Eventually the fermentation stops when the concentration of ethanol is about 12%.- On a large scale, the reaction mixture is cooled. Suggest a reason why this is necessary. (1mk)
….………………………………………………………………………………………………………………………………………………………………………….. - Why does the fermentation stop? Suggest one reasons. (1mk)
….………………………………………………………………………………………………………………………………………………………………………… - What technique is used to concentrate the aqueous ethanol? (1mk)
….……………………………………………………………………………………………………………………………………………………………………………
- On a large scale, the reaction mixture is cooled. Suggest a reason why this is necessary. (1mk)
- A compound X contains carbon, hydrogen and oxygen only. X contains 54.54% of carbon by mass, 9.09% of hydrogen by mass and 36.37% of oxygen by mass. (C=12, O=16, H=1)
- Determine the empirical formula of compound X. (2mks)
- Compound X has a relative molecular mass of 88. Draw the structural formula of compound X. (2mks)
- The table below gives formulae of three organic compounds A, B and C
Giving a reason in each case, select the letter(s) which represent a compound thatCompound Formulae A C2H4O2 B C2H6O C C2H6 - Decolourises acidified potassium manganate (VII). (1mk)
….…………………………………………………………………………………………………………………………………………………………………………………. - Gives effervescence with sodium hydrogen carbonate. (1mk)
….………………………………………………………………………………………………………………………………………………………………………………… - Undergoes substitution reaction with chlorine gas. (1mk)
….………………………………………………………………………………………………………………………………………………………………………………………………………
- Decolourises acidified potassium manganate (VII). (1mk)
- The following is a small reaction of polystyrene polymer. Study it and answer the questions that follow.
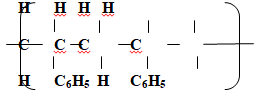
- (Draw the structure of the monomer unit of polystyrene. (1mk)
- Calculate the number of monomers used to form the polystyrene of relative molecular mass of 18096. ( H = 1, C = 12 ) (1mk)
- The fermentation of glucose is catalysed by enzymes from yeast. Yeast is added to aqueous glucose, the solution starts to bubble and becomes cloudy as more yeast cells are formed.
- An experiment was carried out using magnesium ribbon and dilute hydrochloric acid of different concentrations. The time needed to produce 50cm3 of the gas for every experiment was recorded in a table.
Concentration of HCl (moles per litre) 2.0 1.75 1.50 1.25 1.00 0.75 0.50 0.25 Time (seconds) 8.8 10.0 11.7 14.0 17.5 18.7 35.0 70.0 1 ( Sec-1)
Time- Complete the table above for 1/time. (4mks)
- Plot a graph of rate i.e 1/time against concentration. (3mks)
- From your graph determine the concentration needed to produce 50cm3 of hydrogen gas when time is 15.0 seconds (1mks)
…………………………………………………………………………………….…….
…………………………………………………………………………………….……. - From your graph state the relationship between the rate of reaction and concentration. Give a reason. (1mk)
…………………………………………………………………………………….…….
……………………………………………………………………………………… - A state of equilibrium between dichromate (vi) and chromate ions is established as shown below
Cr2O72-(aq) + 2OH-(aq) 2CrO42-(aq) + H2O(l)
Orange (Yellow)- What is meant by dynamic equilibrium? (1mk)
……………………………………………………………………………………………………………………………………………………………………………… - State and explain observation made, when a few pellets of Hydrochloric acid are added to equilibrium mixture ( 2mks)
…………………………………………………………………………………………………………………………………………………………………………………
- What is meant by dynamic equilibrium? (1mk)
-
- The table below shows properties of some elements represented by symbols W,X,Y and Z. Study the information in the table and answer the questions that follows
Element No. Of protons Atomic radius(nm) Boiling point ºC W 2 0.93 -269 X 10 1.31 -246 Y 18 1.54 -186 Z 36 1.89 -152 - Write down the electron arrangement for elements W and X (1mk)
….…………………………………………………………………………………………………………………………………………………………………………………. - In which group of the periodic table are the elements in the table above? Give the name of the group (2mks)
….………………………………………………………………………………………………………………………………………………………………………………….. - Explain why the atomic radius of W is smaller than that of X (1mk)
….…………………………………………………………………………………………………………………………………………………………………………………. - State one use of element X (1mk)
…………………………………………………………………………………………………………………………………………………………………………………
- Write down the electron arrangement for elements W and X (1mk)
- The section below represents part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbol of the elements.
Q X B H M T Y A V Z S - Select the least reactive non-metal. (1mk)
…………………………………………………………………………………….……. - Which of the elements has the greatest tendency of forming covalent compounds in nature? Explain your choice. (1mk)
…………………………………………………………………………………….…….
…………………………………………………………………………………….……. - Explain why the atomic radius of T is smaller than that of M. (2mks)
…………………………………………………………………………………….…….
…………………………………………………………………………………….……. - Compare the electrical conductivity of element X and B. (2mks)
- Select the least reactive non-metal. (1mk)
- The table below shows properties of some elements represented by symbols W,X,Y and Z. Study the information in the table and answer the questions that follows
- Extraction of iron involves two main processes, smelting and refining. Below is the blast furnace which is used to smelt iron from its ore.
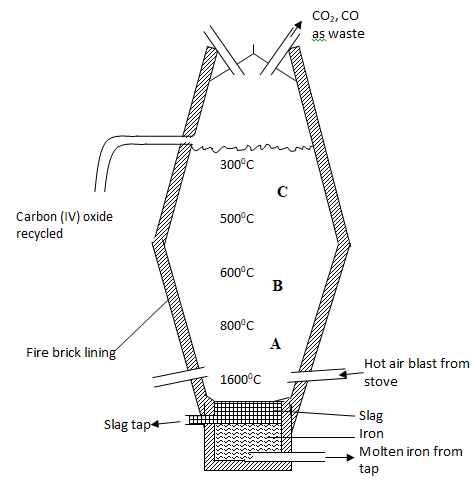
-
- The chief ore is Haematite. Name one other ore used in extraction of iron (1 mark)
…………………………………………………………………………………………………………………………….……………………………………………………… - Name the reducing agent in the process. (1mk)
…………………………………………………………………………………………………………………………….……………………………………………………… - What is the role of the hot air blast in the process? (2mks)
…………………………………………………………………………………………………………………………….………………………………………………………
- The chief ore is Haematite. Name one other ore used in extraction of iron (1 mark)
- Write equations for the reactions that take place at the region marked A, B and C. (3mks)
A………………………………………………………………………
B………………………………………………………………………
C ……………………………………………………………………… - What is the purpose of limestone in the extraction process? (1mk)
……………………………………………………………………………… - Write equations to show how impurities are removed from the ore. (2mks)
…………………………………………………………………………………………………………………………….…………………………………………………………………………………………………………………………….…………………… - State one environmental effect of the process. (1mk)
……………………………………………………………………………………………………………………………………………………………………………………
-
-
- Read the following passage and answer the questions.
A salt K was heated with slaked lime (calcium hydroxide). A colourless gas L with a characteristic smell and turns red litmus paper blue was evolved. A large quantity of this gas was passed through an inverted filter funnel into Copper(II)sulphate solution, and a deep blue solution M was obtained.- Identify gas L (1mk)
….………………………………………………………………………………………………………………………………………………………………………………… - What is K most likely to be? (1mk)
….………………………………………………………………………………………………………………………………………………………………………………… - Write an equation for the reaction between K and slaked lime (1mk)
….…………………………………………………………………………………….. - Write an ionic equation for the reaction with copper(II) sulphate forming the deep blue solution (1mk)
….…………………………………………………………………………………………………………………………………………………………………………
- Identify gas L (1mk)
- Study the flow chart below and answer questions that follow:
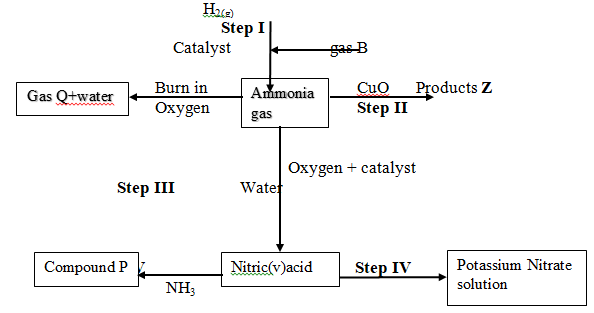
- State one source of gas B (1mk)
………………………………………………………………………………………………….………………………………………………………………… - Name the catalysts used in; (1mk)
- Step I
………………………………………………………………………………… - Step III
…………………………………………………………………………………
- Step I
- Write chemical equations for reactions in; (3mks)
- Step I
- Step II
- Step V
-
Identify any other gas that can be used instead of Ammonia in step II (1mk)
………………………………………………………………………………… -
State one use of gas Q
- State one source of gas B (1mk)
- Read the following passage and answer the questions.

MARKING SCHEME
-
- Consider the following reaction:
A2(g) + B2(g) 2AB(g), ΔH = +75 kJ
Sketch an energy level diagram showing the relative activation energies for the catalysed and uncatalysed reactions using the axes below. (2mks)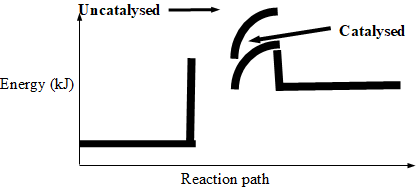
- Given that; ΔHf (Al2O3) = – 1590 kJmol-1
ΔHf(Cr2O3) = -1134kJmol-1
Calculate the heat of reaction for; (2mks) - The following data was obtained during an experiment
Mass of ethanol burnt = 0.2g
Mass of water in the calorimeter = 200g
Specific heat capacity of water = 4.2 jg-1k-1
Initial temperature of water = 23.5ºC
Final temperature of water = 28.0ºC- How was the mass of ethanol that burnt determined? (1mk)
By sybtracting the mass of the burner and ethanol before igniting and the mass of the burner and ethanol after the burning - How much heat was required to raise the temperature of water from 23.5 ºC to 28.0ºC? (2mks)
Heat produced ∆H = mass of water(m) x specific heat capacity (c)x∆T
=> 200 x 4.2 x 4.5 = 3780 Joules = 3.78 kJ - Two assumptions were made in calculating the enthalpy of combustion for ethanol. State them. (1mk)
No heat loss to the environment
The calorimeter did not absorb any heat - Determine the molar enthalpy of combustion of ethanol.(C= 12,H=1, O=16) (2mks)
Moles of ethanol used = 0.2/46
=0.0043 moles 3.78kJ
1 Mole ?
= 3.78x1/0.0043
= -879.069kJ/mol - Write a thermochemical equation for the combustion of ethanol given the accurate value for enthalpy of combustion is – 1368 kJmol-1. (1mk)
C2H5OH +3O2(g) →2CO2(g)+3H20(l) – 1368 kJmol-1.
- How was the mass of ethanol that burnt determined? (1mk)
- Consider the following reaction:
- Two half cells were connected as shown to form a voltaic cell. The reduction potentials are given.

Pb2+(aq) + 2e Pb(s)EӨ = – 0.13V
Fe2+(aq) + 2e Fe(s)EӨ = – 0.44V- Calculate the e.m.f of the cell. (1mk)
EREDOX=ERED-EOX = -0.13--0.44
=+0.31V - Sodium Chloride is used as the salt bridge.State the two functions of the salt bridge. (2mks)
complete the circuit
maintain balance of charges /ions on both half cells. - Show the direction of the electron flow in the external circuit. (1mk)
- The e.m.f of the cell will reduce with time. Give a reason for this. (1mk)
- During electrolysis of water acidified with Sulphuric acid, two gases were produced at the electrodes:
- State which ions are preferentially discharged at the electrodes. Explain with aid of half ionic equations.
Anode. (2mks)
OH-(aq) from water (H2O)
4OH-(aq) 2H2O(l)+O2(g)+4e
OH-ions selectively discharged instead of SO42- ions at the anode
Cathode. (2mks)
H+(aq) from either sulphuric(VI) acid (H2 SO4) or water (H2O)
H+(aq)+4e → 2H2(g) - Calculate the volume of the gases at s.t.p produced when a current of 0.025A is passed for 4 hours. (1 Faraday=96500C) (3mks)
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 0.025x (4x 60 x 60)
= 360C
ANODE; 4 moles of electrons = 4 Faradays = 96500 x 4 C 22400cm3
360C -> 360 x 22400
96500 x 4
=20.89cm3
CATHODE; ratio 1;2
20.89x2=41.78cm3
- State which ions are preferentially discharged at the electrodes. Explain with aid of half ionic equations.
- Calculate the e.m.f of the cell. (1mk)
-
- The fermentation of glucose is catalysed by enzymes from yeast. Yeast is added to aqueous glucose, the solution starts to bubble and becomes cloudy as more yeast cells are formed.
C6H12O6(aq) → 2C2H5OH(aq)+2CO2(g)
The reaction is exothermic. Eventually the fermentation stops when the concentration of ethanol is about 12%.- On a large scale, the reaction mixture is cooled. Suggest a reason why this is necessary. (1mk)
kil yeast or denature enzymes (due to increase in temperature) - Why does the fermentation stop? Suggest one reasons. (1mk)
- All glucose used up
- yeast killed or denatured or damaged by ethanol/alcohol
- What technique is used to concentrate the aqueous ethanol? (1mk)
Fractional distillation
- On a large scale, the reaction mixture is cooled. Suggest a reason why this is necessary. (1mk)
- A compound X contains carbon, hydrogen and oxygen only. X contains 54.54% of carbon by mass, 9.09% of hydrogen by mass and 36.37% of oxygen by mass. (C=12, O=16, H=1)
- Determine the empirical formula of compound X. (2mks)
Element C H O Mass 54.54 9.09 36.37 R.A.M 12 1 16 Moles 54.54/12= 4.545 9.09/1= 9.09 36.37/16 = 2.273 Mole ratio 4.545/2.273
29.09/2.273
3.999>>42.273/2.273
1C2H4O - Compound X has a relative molecular mass of 88. Draw the structural formula of compound X. (2mks)
(C2H4O)n=88
(12 x 2 +1 x 4 +16)n=88
44n=88
N=2
(C2H4O)2= C4H8O2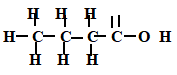
- Determine the empirical formula of compound X. (2mks)
- The table below gives formulae of three organic compounds A, B and CH
Giving a reason in each case, select the letter(s) which represent a compound thatCompound Formulae A C2H4O2 B C2H6O C C2H6 - Decolourises acidified potassium manganate (VII). (1mk)
B Its an alkanol hence oxidized to alkanoic acid - Gives effervescence with sodium hydrogen carbonate. (1mk)
A Reacts to produce CO2 - Undergoes substitution reaction with chlorine gas. (1mk)
C its saturated
- Decolourises acidified potassium manganate (VII). (1mk)
- The following is a small reaction of polystyrene polymer. Study it and answer the questions that follow.
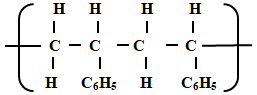
- Draw the structure of the monomer unit of polystyrene. (1mk)
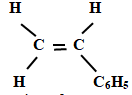
- Calculate the number of monomers used to form the polystyrene of relative molecular mass of 18096. ( H = 1, C = 12 ) (1mk)
18096/104
= 174 monomers
- Draw the structure of the monomer unit of polystyrene. (1mk)
- The fermentation of glucose is catalysed by enzymes from yeast. Yeast is added to aqueous glucose, the solution starts to bubble and becomes cloudy as more yeast cells are formed.
- An experiment was carried out using magnesium ribbon and dilute hydrochloric acid of different concentrations. The time needed to produce 50cm3 of the gas for every experiment was recorded in a table.
Concentration of HCl (moles per litre) 2.0 1.75 1.50 1.25 1.00 0.75 0.50 0.25 Time (seconds) 8.8 10.0 11.7 14.0 17.5 18.7 35.0 70.0 1 ( Sec-1)
Time0.1140 0.1000 0.0854 0.0714 0.0571 0.0534 0.0286 0.0143 1 x 10-2
t11.4 10.0 8.54 7.14 5.71 5.34 2.86 1.43 - Complete the table above for 1/time. (4mks)
- Plot a graph of rate i.e 1/time against concentration. (3mks)
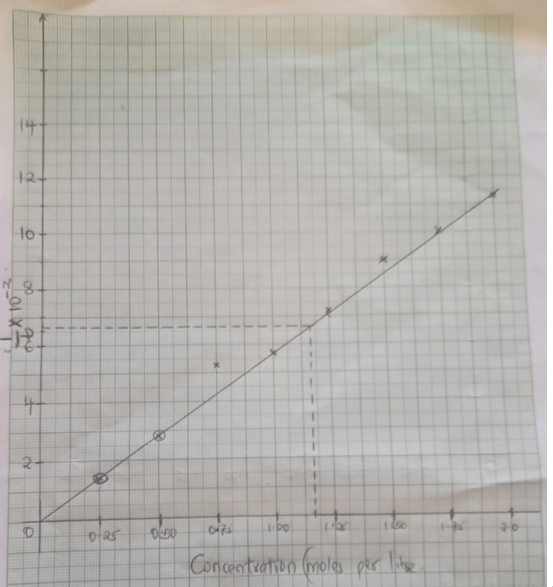
- From your graph determine the concentration needed to produce 50cm3 of hydrogen gas when time is 15.0 seconds (1mks)
T=15
1 = 0.0667
15
= 1.1625 - From your graph state the relationship between the rate of reaction and concentration. Give a reason. (1mks)
Rate of reaction increases with increase in concentration - A state of equilibrium between dichromate (vi) and chromate ions is established as shown below
Cr2O72-(aq) + 2OH-(aq) 2CrO42-(aq) + H2O(l)
Orange (Yellow)- What is meant by dynamic equilibrium? (1 mk)
a reaction in which the rate of forward reaction is equal to the rate of backward reaction
balance of the rate of formation of products and reactants. - State and explain observation made, when a few pellets of Hydrochloric acid are added to equilibrium mixture (2 mks)
solution mixture makes it to be more Orange in colour.
Hydrochloric acid/ H+ (aq) is added to the equilibrium mixture a stress is created on the reactant side on the OH- (aq). H+ ions react with OH- (aq) to form water.
The equilibrium shift backward to the left to add/replace the 2OH- (aq) that have reacted with the H+ (aq) ions .More Cr2O7(aq)ions formed in the
- What is meant by dynamic equilibrium? (1 mk)
-
- The table below shows properties of some elements represented by symbols W,X,Y and Z. Study the information in the table and answer the questions that follows
Element No. Of protons Atomic radius(nm) Boiling point ºC W 2 0.93 -269 X 10 1.31 -246 Y 18 1.54 -186 Z 36 1.89 -152 - Write down the electron arrangement for elements W and X (1mk)
W 2
X 2:8 - In which group of the periodic table are the elements in the table above?Give the name of the group (2 mk)
Group VII
Halogens - Explain why the atomic radius of W is smaller than that of X (1mks)
X has more/2 energy levels than W (1 energy levels). - state one use of element X (1mk)
Use in making neon advertising coloured signs
Used to make high voltage indicators
Neon and helium are used in making gas lasers
Liquid helium is an economical refrigerant
- Write down the electron arrangement for elements W and X (1mk)
- The section below represents part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbol of the elements.
Q X B H M T Y A V Z S - Select the most reactive non-metal. (1mk)
M - Which of the elements has the greatest tendency of forming covalent compounds in nature? Explain your choice. (1mk)
Q or H,group IV ,have a valency of 4 hence can share its 4 pairs of electrons - Explain why the atomic radius of T is smaller than that of M. (2mks)
T has more protons(18) hence higher nuclear charge than M (17 protons) attracting outermost electrons closer to the nucleaus reducing the atomic radius - Compare the electrical conductivity of element X and B. (2mks)
B has higher conductivity,it has 3 delocalised electrons X has 1 delocalised electrons
- Select the most reactive non-metal. (1mk)
- The table below shows properties of some elements represented by symbols W,X,Y and Z. Study the information in the table and answer the questions that follows
- Extraction of iron involves two main processes, smelting and refining. Below is the blast furnace which is used to smelt iron from its ore.

-
- The chief ore is Haematite. Name one other ore used in extraction of iron (1 mark)
Magnetite / Siderite - Name the reducing agent in the process. (1 mk)
Carbon(II)oxide - What is the role of the hot air blast in the process?(2 mks)
Reacts with coke/charcoal/carbon to form carbon(IV)oxide gas
raises the temperature at the bottom of the furnace to about 2000K(1650ºC).
- The chief ore is Haematite. Name one other ore used in extraction of iron (1 mark)
- Write equations for the reactions that take place at the region marked A, B and C. (3 mks)
- C(s)+O2(g) → CO2(g)
- Fe2O3(s)+3CO(g) →2Fe(s)+CO2(g)
Fe3O4(s)+4CO(g) →3Fe(s)+4CO2(g) - CaCO3(s) →CaO(s) + CO2(g)
- What is the purpose of limestone in the extraction process? (1 mk)
decomposes to quicklime /calcium oxide which reacts to remove impurities and produce more carbon(IV)oxide gas. - Write equations to show how impurities are removed from the ore.(2 mks)
CaO(s) + SiO2(s)→ CaSiO3(l)
CaO(s) + Al2O3(s)→CaAl2O4(l) - State one environmental effect of the process. (1mk)
- Carbon(IV)oxide(CO2) gas is a green house gas that causes/increases global warming if allowed to escape/leak from the furnace.
- Carbon(II)oxide(CO)gas is a highly poisonous/toxic odourless gas that can kill on leakage. It is preferentially absorbed by the haemoglobin in mammals instead of Oxygen to form a stable compound that reduce free hemoglobin in the blood.
- Haematite (Fe2O3), Magnetite(Fe3O4) and Siderite (FeCO3) are extracted through quarrying /open cast mining that cause soil / environmental degradation .
-
-
- Read the following passage and answer the questions.
A salt K was heated with slaked lime (calcium hydroxide). A colourless gas L with a characteristic smell and turns red litmus paper blue was evolved. A large quantity of this gas was passed through an inverted filter funnel into Copper(II)sulphate solution, and a deep blue solution M was obtained.- Identify gas L (1 mk)
Ammonia - What is K most likely to be? (1 mk)
Ammonium chloride - Write an equation for the reaction between K and slaked lime (1 mk)
Ca(OH)2(s)+NH4Cl(s)→CaCl2(aq)+H2O(l)+2NH3(g) - Write an ionic equation for the reaction with copper(II)sulphate forming the deep blue solution (1 mks)
Cu(OH)2(s) + 4NH3(aq) → [Cu(NH3)4]2+(aq)+2OH–(aq)
- Identify gas L (1 mk)
- Study the flow chart below and answer questions that follow:

- State one source of gas B (1 mk)
Fractional distillation of liquid air - Name the catalysts used in; (1 mk)
- Step I
Finely divided Iron - Step III
Platinum or platinum rhodium
- Step I
- Write chemical equations for reactions in; (3 mks)
- Step I
N2(g)+3H2(g)→ 2NH3(g) - Step II
2NH3(g)+3CuO(s)→N2(g)+3H2O(l)+3Cu(s) - Step V
NH3(aq) + HNO3(aq)→NH4NO3(aq)
- Step I
- Identify any other gas that can be used instead of Ammonia in step II (1 mk)
Hydrogen/Carbon(II)oxide - State one use of gas Q (1mk)
- Used in the Haber process in the manufacture of ammonia.
- Due to its inert nature, it is mixed with argon to fill electric bulbs (to avoid soot formation).
- In liquid state it is used as an inert refrigerant e.g. storage of semen for artificial insemination.
- Due to its inert nature, it is used in food preservation particularly for canned products i.e. it prevents combination of oxygen and oil which tends to enhance rusting.
- It is used in oil field operation called enhanced oil recovery where it helps to force oil from subterranean deposits
- State one source of gas B (1 mk)
- Read the following passage and answer the questions.
Download Chemistry Paper 2 Questions and Answers - Kassu Joint Mock Examination 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students