Instructions to students:
- Answer all questions
- Candidates must answer all questions in English
-
- Use the standard reduction potentials given below to answer the questions that follow.
Half –cell reactions Eθ(V)
B2+(aq) + 2e−B(S) −0.44
A2+(aq) + 2e−A(S) −0.76
C2(aq) + 2e−2C−(S) +0.54
2E+(aq) + 2e−B(S) 0.00
D3+(aq) + 3e−D(S) −1.66
- Identify the strongest oxidizing agent. Give a reason. (1mk)
- Arrange the elements having the negative standard reduction potentials in order of increasing reactivity. (2mks)
- Calculate the e.m.f of a cell formed by combing the half-cells of B and D. (2mks)
- Write down the overall equation for the reaction taking place in (iii) above. (1mk)
- Draw an electrochemical cell represented by the cell notation A(s) / A2+(aq) // B2+/B(S). (3mks)
- Draw a well – labeled diagram to showing how a spoon made of D is electroplated using A. (3mks)
- Determine the oxidation state of Mn in MnO4- (1mk)
- Use the standard reduction potentials given below to answer the questions that follow.
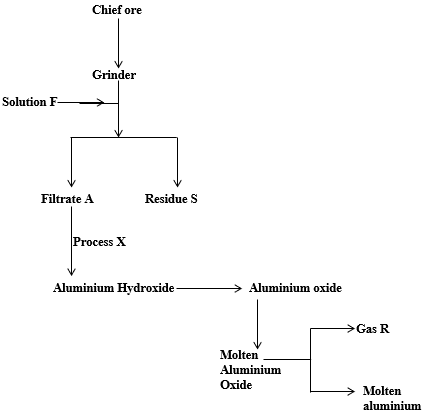
- Study the flow chart below for the extraction of aluminum from ore.
- Name the chief ore from which aluminium is extracted. (½ mk)
- Give two impurities present in the ore. (1mk)
- Briefly explain how the impurities in (ii) above are removed. (2mks)
- Identify: (1mk)
- Solution F…………………………………………………………………………………….
- Residue S…………………………………………………………………………………….
- Write balance equations for
- The reaction taking place in process X (1mk)
- Reaction producing gas F (1mk)
- State any one uses of aluminium (½ mk)
- Compare the atomic radius of aluminium with that of sodium. (Al = 13, Na = 11). (2mks)
- Aluminium reacts with chlorine to form molecular compound. Using dots (•) and (X) to represent electrons. Show the bonding in the compound formed. (Al = 13, Cl = 17) (2mks)
-
-
- Define the term “Dynamic Equilibrium”. (1mk)
- List two factors, other than concentration, that affects chemical equilibrium. (1mk)
- Explain the importance of the factors mentioned above to an industrialist. (1mk)
- Consider the reaction below an equilibrium .
Br2(g) + H2O(l)2H+(aq) + OBr(aq) +Br(aq)
(Orange) (Colourless)
State and explain the observation made when sodium hydroxide solution is added to the equilibrium mixture. (2mks)
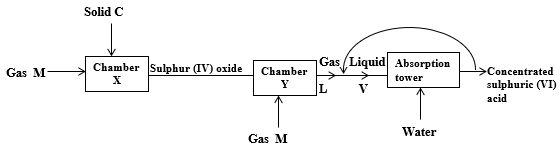
- The flow chart below shows some of the process involved in the large scale manufacture of sulphuric (VI) acid. Study and use if to answer the questions that follow.
- Why is the process referred to as Contact Process? (½mk)
- Identify (2mks)
- Solid C………………………………………………………………………………………..
- Gas M…………………………………………………………………………………………
- Gas L………………………………………………………………………………………….
- Liquid V ……………………………………………………………………………………...
- Write down the equation for the reaction taking place in the absorption tower. (1mk)
- Other than recycling, describe how pollution can be minimized in the process. (1mk)
- 15,000 litres of suphuric (IV) oxide is reacted in chamber Y. With an efficiency of 80% determine the final mass of sulphuric (VI) acid produced in Kg. (2 ½mks)
(MGV= 24 L)
-
-
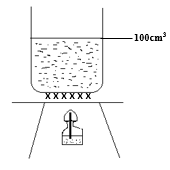
- In an experiment to determine the heat of methanol, a student set-up the apparatus as shown below. Study the set-up and use the data obtained to answer the questions that follow.
Initial temperature of water = 22ºC
Finial temperature of water = 36ºC
Initial mass of lamp + methanol = 85.10 g
Final mass of lamp + methanol = 84.75 g
Density of water = 1g/cm3 C = 4.2 Jg-1k-1- Determine the heat change for this experiment. (1mk)
- Determine the molar enthalpy of combustion of methanol. (2mks)
- Write down a thermochemical equation for the reaction. (1mk)
- Draw an energy level diagram for the reaction. (2mks)
- The molar enthalpy of combustion of thane (C2H6) is -1560 kJmol-1. Explain which one is a better fuel between methanol and ethane.
(C= 12, O = 16, H = 1) (2mks)
- Use the information below to answer the question that follow
Ca (s) + ½ O2(g) → CaO(s) ∆H1 = - 635kJmol-1
C(s) + O2(g) → CO2(g) ∆H2 = -394kJmol-1
Ca(s) + C(s) + 3/2O2(g) → CaCO3(S) ∆H3 = - 207kJmol-1- Name two enthalpy changes represented by ∆H2 (2mks)
- Use the information above to calculate the enthalpy change for the reaction (3mks)
CaO(s) + CO2(g) → CaCO3(s)
- In an experiment to determine the heat of methanol, a student set-up the apparatus as shown below. Study the set-up and use the data obtained to answer the questions that follow.
-
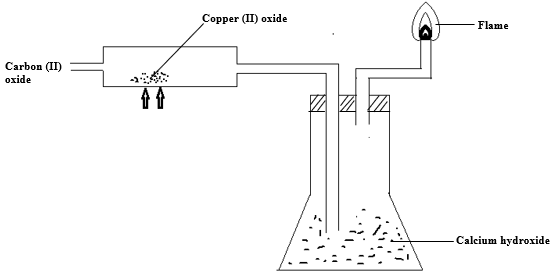
- Study the set-up below and use it to answer the questions that follow.
- State the observation made in the combustion tube. (1mk)
- Write an equation for the reaction producing the flame. (1mk)
- Explain the observation made in the conical flask when the experiment is done for a longer period of time (2mks)
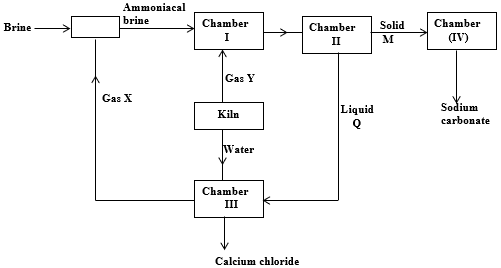
- The flow chart below show the manufacture of sodium carbonate. Study it carefully and answer the questions that follow.
- Identify (1 ½ mks)
- Gas X……………………………………………
- Gas Y…………………………………………….
- Liquid Q …………………………………………
- Give one use of calcium chloride. (½ mk)
- Write balanced chemical equations for the reactions in (2mks)
- Chamber III
- Chamber IV
- Give two reasons why the Solvay process is considered one of the most efficient industrial process? (2mks)
- Name two process taking place in chamber II. (1mk)
- Identify (1 ½ mks)
- Study the set-up below and use it to answer the questions that follow.
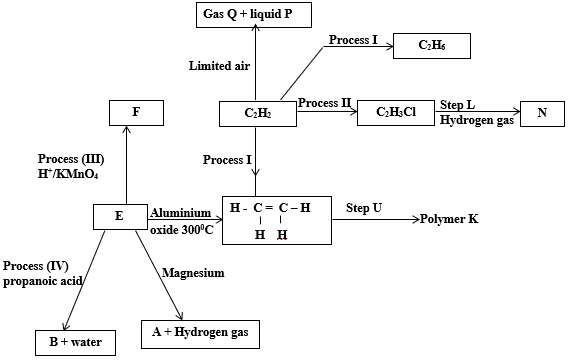
- Study the flow chart below and use it to answer the questions that follow.
- Give the names of substances (2mks)
- N…………………………………………………………………………………………………..
- F…………………………………………………………………………………………………..
- A………………………………………………………………………………………………….
- E…………………………………………………………………………………………………..
- Give the conditions necessary for: (1 ½mks)
- Process I
- Process IV ( ½mk)
- Draw the open structural formula of B. (1mk)
- Write balanced chemical equations for the reaction in
- Process II. (1mk)
- Between E and magnesium. (1mk)
- Name: (1 ½ mks)
- Process (I)………………………………………………………………………………………
- Process (III)……………………………………………………………………………………….
- Process (IV)………………………………………………………………………………………
- Give one distinguishing property of substance B. (½mk)
- State two ways in which polymers K has improved human life. (2mks)
- Give the names of substances (2mks)
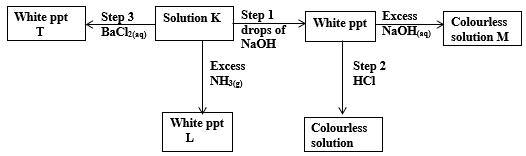
- Study the flow chart below and use it to answer the questions that follow.
- Identify the anion and cation in solution K
- Name the white precipitate L. (1mk)
- Name the type of reaction in step 2. (1mk)
- Name any other solution that would form white ppt T in step 3. (1mk)
- Write the formula of the complex ion in solution M. (1mk)
- Describe how a dry sample of the salt forming the white ppt T can be prepared in the laboratory starting with barium oxide. (3mks)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions - Mokasa II Joint Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students