CHEMISTRY
Questions
- Differentiate between the following terms as used in chemistry.
- Temporary change and physical change. (1mk)
- Atomic number and mass number. (1mk)
- Ionization energy and electron affinity. (2mks)
- Is air a mixture or a compound? Explain. (2mks)
- Oxygen can be prepared from the decomposition of hydrogen peroxide in the presence of a catalyst.
- Name the catalyst used. (1mk)
- Write an equation for the formation of oxygen gas. (1mk)
- State the simple test for oxygen gas. (1mk)
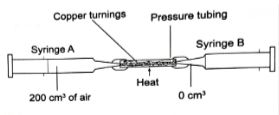
- The apparatus below were used to determine the volume of oxygen in air. About 200cm3 of air was passed repeatedly from syringe A to syringe B over heated copper turnings as shown in the diagram. After sometime, the volume of air in the syringe A was 160cm3 and syringe B 0cm3.
- Write a chemical equation for the reaction that took place in the combustion tube. (1mk)
- Calculate the percentage of oxygen in the initial sample of air. (3mks)
- State two possible sources of errors in the experiment. (2mks)
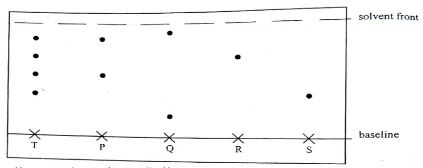
- An experiment was carried out to determine the presence of substance P, Q, R and mixture T. The results obtained are shown in the figure below.
- Name the method of separation illustrated above. (1mk
- Select :
- One substance which contains a component not present in T. (1mk)
- A substance which is least soluble in the solvent used .(1mk)
- Give the valencies of the cation and anion in each of the following compounds:
- Zinc sulphate (1mk)
- Magnesium carbonate (1mk)
- Aluminum nitrate (1mk)
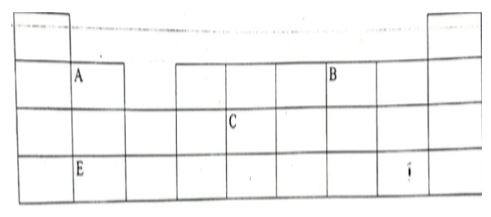
- The diagram below represents a grid that is part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.
- Write the electron arrangement of element C. (1mk)
- On the grid provided, show with a tick (✅) the position of the element D whose atomic number is 18 (1mk)
- Element E is more reactive than A. Explain. (1mk)
- State any two uses of element D.(2mks)
- Write a balanced chemical equation for the reaction of element A and element B. (1mk)
- Use dot (•) and cross (X) diagram draw the structure of the compound formed between the reaction of element A and element B. (2mks)
- Explain the following observations :
- The melting point of sodium is higher that of potassium (2mks)
- Sodium chloride solution conducts electric current while sugar does not (2mks)
-
- Define the term salt (1mk)
- Write an ionic equation between magnesium sulphate and lead (ll) nitrate solution. (2mks)
- Describe an experiment on how you can use the above (a) reactants to prepare a sample of lead (ll) sulphate powder. (4mks)
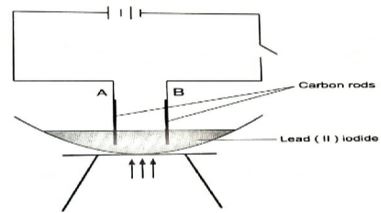
- The set up below was used to investigate the effect of electric current on the binary electrolyte (molten lead (ll) iodide)
- What is a binary electrolyte? Give another example (2 mks)
- Identify the cathode and anode. Explain (2mks)
- State what is observed at the:
- cathode (1mk)
- Anode (1mk)
- Write the half equations for the reactions at the :
- Anode (1mk)
- Cathode (1mk)
- State any two applications of the process investigated above. (2mks)
- Define the term allotropy
- Name two main allotropes of carbon. (2mks)
- Give any three uses of carbon (3mks)
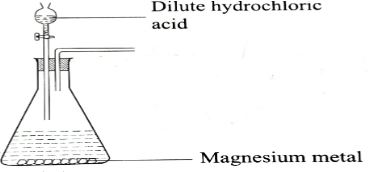
- A student at Victory Boy's High School wanted to prepare dry carbon (IV)oxide.
- Identify and correct appropriately one mistake in the set up. (1mk+1mk)
- Complete the set up to show how dry carbon (IV) oxide gas may be prepared and collected. (3mks)
- Write a balance chemical equation for the reaction that occurred in the conical flask for the preparation of carbon (IV) oxide. (1mk)
- Describe the chemical test of carbon (IV) oxide gas. (2mks)
- Give two uses of carbon (IV) oxide (2mks)
- What is the charge and oxidation number of the following ions.
- Copper (l) ion.
Charge.................................... (1mk)
Oxidation number................................ (1mk) - Lead (ll) ion
Charge........................ (1mk)
Oxidation number...................... (1mk) - Sulphide ion.
Charge......................................... (1mk)
Oxidation number......................... (1mk)
- Copper (l) ion.
- Element K (not actual symbol of element) has isotopes with relative abundances as shown below.
Calculate the relative atomic mass of element K (3mks)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions - Form 3 Opener Term 1 Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students