INSTRUCTIONS TO STUDENTS
- Answer all questions in this question paper.
-
- State and explain simple method you can use to separate a mixture of sulphur powder and iron fillings. (2mark)
- A mixture of iron and sulphur was heated strongly until it glowed red throughout and then left to cool. Explain why you cannot obtain sulphur and iron from the product using the method you stated in (a) above (2marks)
- Explain why the following substances are good conductors of electricity:
- Molten lead II bromide (1mark)
- Aluminium (1mark)
- Define the term electrolyte (1mark)
- The following set up was used to investigate the effect of an electric current on silver chloride
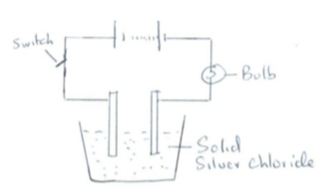
- Label the cathode and anode on the diagram (1mk)
- When the switch was closed the bulb did not light. Explain. (1mk)
- If the bulb lights, write the equation of the reaction occurring at the cathode. (1mk)
- State and explain the observation made at the anode. (2mks)
- State three application of electrolysis. (3marks).
- Calculate the pressure required to compress 4.24 dm3 of a gas at 5.4299 X 104Pascal’s to 1.56 dm3 at constant temperature. (2marks)
- Draw the structure of;
- Hydroxonium ion H3O+ (2mk)
- Ammonium ion (Al = 13, 0 = 8) (2mk)
- The table below shows some properties of substances A-E. Study it and answer the questions that follow.
Substance Solubility in water Solubility in chloroform m.p. (°C) b.p.(°C) A soluble soluble −22 141 B insoluble soluble 115 444 C soluble insoluble 801 1465 D insoluble soluble −188 −42 E insoluble insoluble 1083 2600 - Which of the substances is a gas at room temperature of 25 ᵒC? . (1mark)
- What is the physical state of substance A at room temperature of 25 ᵒ C? . (1mark)
- How can you separate the mixture of substances B , C and E ? (3marks)
- What volume of acidified potassium manganate VII of concentration 0.02 moles per dm3 is decolorized by 200 dm3 of hydrogen peroxide of concentration 0.02 moles per dm3 ? (3marks)
Use the following ionic equation
2MnO4- (aq) + 6H+ (aq) + 5H2O2(aq)2Mn2+ (aq) + 8H2O(l) + 5O2(g)
- A mass of 3.6 g magnesium reacts in excess chlorine to form a chloride. If the mass of the chloride formed is 14.25 g, find the formula of the chloride formed. (Mg = 24, Cl =35.5 ). (2marks)
- Starting with copper metal describe how a dry sample of copper II carbonate can be prepared in the laboratory. (3 marks).
- The table below shows the first ionization energies of metals A to D (not their actual chemical symbols) in the same group of the periodic table.
Metal A B C D First ionization energy (kJmol-1) 402 496 520 419 - Arrange the metals in order of that they occur in the periodic table starting from the topmost to the lowest. Give a reason to support your answer. (3marks)
- Which of the metals has the largest atomic radius? (1mark)
- When a piece of calcium is dropped into a beaker of water, it sinks to the bottom and bubbles of a gas are observed on the surface of the metal.
- Why does calcium sink to bottom of the beaker? (1mark)
- Name the gas that is formed in the reaction. (1mark)
- Besides effervescence, what else is observed in the beaker as the reaction progresses?Explain this observation. (2marks)
- Write an equation for the reaction between calcium and water. 1mk
- Explain the following statements:
- Following a bee’s sting, application of sodium hydrogen carbonates to the affected area of the skin reliefs the irritation. (2marks)
- It is not advisable to clean aluminium utensils using wood ash . (2marks)
- The set-up below was used to investigate some properties of hydrogen gas. Study it and answer the questions that follow:
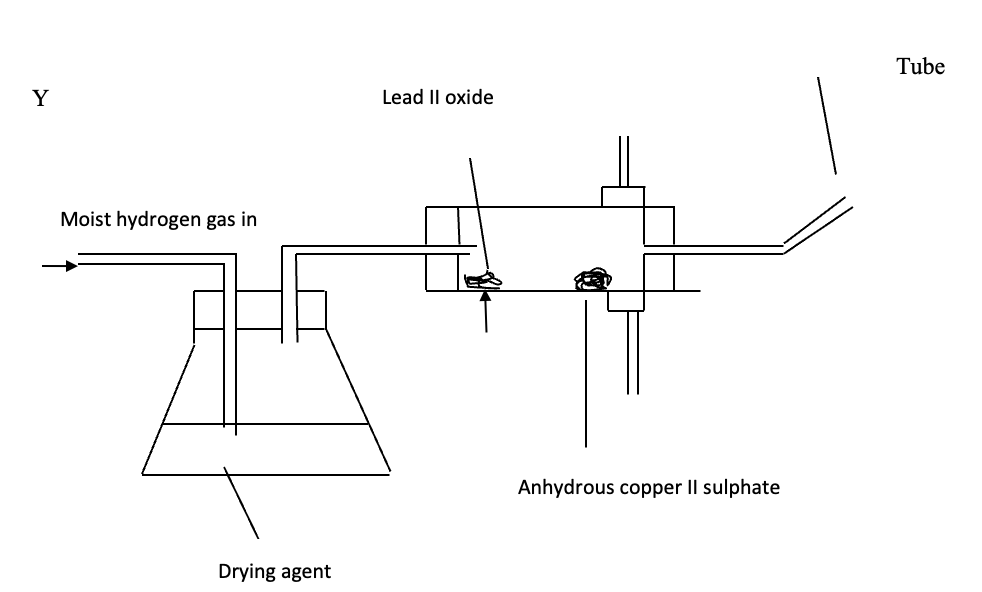
- Name a suitable liquid that can serve as a drying agent. (1mark)
- State the observations you would expect in the combustion tube as the experiment progresses. (2mks)
- Explain the following terms:
- Water of crystallization (1mark)
- Hygroscopy (1mark)
- Acidic salts (1mark)
- Normal salts (1mark)
- Explain the role of helium in the welding of metals. (2marks)
- Whereas hydrogen was commonly used in airships and weather balloons earlier on it is no longer used nowadays. Give a reason for this. (1mark)
- Chebet, Mutua and Waweru are international athletes. Paper chromatography was used to test for the presence of illegal drugs in their blood which enhance the performance. The diagram below shows the chromatogram with the illegal drug labeled N.
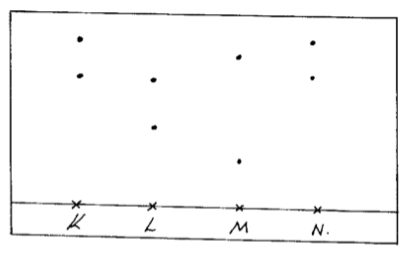
- Who among them tested positive for the illegal drug? Explain. (2marks)
- Explain what is meant by ‘solvent front’. (1mark)
- An aqueous solution of ammonia was added drop wise to a solution of copper (II) Sulphate until in excess .State the observation made when
- A few drops of aqueous ammonia were added. (1 mark)
- Excess aqueous ammonia was added. (1 mark)
- The table below gives information about the ions W + and y2-
Ion W+ Y2− Electrons arrangements 2.8 2.8.8 Number of neutrons 12 16 - How many protons are there in the nucleus of
- Elements W? (1 mark)
- Elements Y? (1 mark)
- Write the formula of the compound formed when W and Y reacts. (1 mark)
- State two conditions under which the compound would conduct electricity. (2marks)
- How many protons are there in the nucleus of
- The following data gives the PH vales of some solution A, B and C.
Solution PH A 13.0 B 6.9 C 2.0 - Which solution would produce carbon (IV) oxide gas when reacted with copper (II) carbonate? Explain. (2 mark)
- What colour change would occur in solution A on addition of three drops of phenolphthalein indicator. (1 mark)
- What volume of 0.2 M hydrochloric acid would react completely with 0.005 moles of pure calcium carbonate? (3 marks)
- What is an allotrope? (1mark)
- Give two allotropes of sulphur. (2marks)
- State three uses of sulphur. (3marks)
MARKING SCHEME
-
- State and explain simple method you can use to separate a mixture of sulphur powder and iron fillings (2mark)
- Use of magnet, iron is magnetic thus will be attracted by magnet while sulphur is non- magnetic and will not be attracted by magnet
- A mixture of iron and sulphur was heated strongly until it glowed red throughout and then left to cool. Explain why you cannot obtain sulphur and iron from the product using the method you stated in (a) above (2marks)
- A compound FeS is formed.
- State and explain simple method you can use to separate a mixture of sulphur powder and iron fillings (2mark)
- Explain why the following substances are good conductors of electricity:
- Molten lead II bromide (1mark)
- The ions are free to move
- Aluminium (1mark)
- Has delocalized electrons
- Molten lead II bromide (1mark)
- Define the term electrolyte (1mark)
- A substance that conducts electricity in molten or in aqueous form and get decomposed
- The following set up was used to investigate the effect of an electric current on silverchloride
- Label the cathode and anode on the diagram (1mk)
- When the switch was closed the bulb did not light. Explain. (1mk)
- Solid silver chloride cannot conduct electric current since ions are fixed
- If the bulb lights, write the equation of the reaction occurring at the cathode. (1mk)
2Ag+ + 2e− → 2Ag - State and explain the observation made at the anode. (2mks)
- Green yellow substance is formed. Cl ions is oxidized at the anode to Cl 2 gas which is green yellow
- State three application of electrolysis. (3marks).
- Coating metal with other metals
- Manufacture of pure substances
- Extractions of metals i.e sodium
- Calculate the pressure required to compress 4.24 dm3 of a gas at 5.4299 X 104 Pascal’s to 1.56 dm3 at constant temperature. (2marks)
P1V1 =P2V2
4.24dm3 × 5.4299 × 104Pa = 1.56x
x = 4.24dm³ × 5.4299 × 104Pa
1.56
= 147581.89Pa - Draw the structure of;
- Hydroxonium ion H3O+ (H=1, O=8). (2mk)
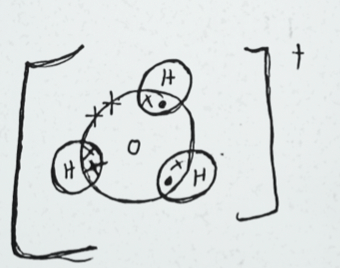
- Ammonium ion (N = 7, H = 1) (2mk)

- Hydroxonium ion H3O+ (H=1, O=8). (2mk)
- The table below shows some properties of substances A-E. Study it and answer the questions that follow.
- Which of the substances is a gas at room temperature of 25 ᵒC? . (1mark)
- D
- What is the physical state of substance A at room temperature of 25 ᵒ C? . (1mark)
- Liquid
- How can you separate the mixture of substances B , C and E ? (3marks)
- Add chloroform to the mixture to dissolve B√1 . Filter and evaporate the to obtain. Dry the residue to obtain E. √1
- Mixture to obtain B√ 1 . Add water to the residue to dissolve C , filter and evaporate the mixture to obtain. Dry the residue to obtain E.
- Which of the substances is a gas at room temperature of 25 ᵒC? . (1mark)
- What volume of acidified potassium manganate VII of concentration 0.02 moles per dm3 is decolorized by 200 dm3 of hydrogen peroxide of concentration 0.02 moles per dm3 ? Use the following ionic equation. (3marks)
2MnO4- (aq) + 6H+ (aq) + 5H2O2(aq)2Mn2+ (aq) + 8H2O(l) + 5O2(g)
1000cm3 of H2O4− = 0.02moles
200cm3 = x
x = 200 × 0.02
1000
= 0.004 moles
Mole ratio = 5 : 2
Moles of MnO4- = 0.004 × 2
5
= 0.04moles - A mass of 3.6 g magnesium reacts in excess chlorine to form a chloride. If the mass of the chloride formed is 14.25 g, find the formula of the chloride formed. (Mg = 24, Cl =35.5 ). (2marks)
Mass of Mg = 3.6g
Mass of Cl = 14.25 − 3.6 = 10.65g ✓½
Element Mg Cl
Mass 3.6 10.65
Mole ratio 3.6/24 = 0.15 10.65/35.5 = 0.3 ✓½
Ratio of atoms. 0.15/0.15 =1 0.3/0.15 =2. ✓½
E. formula MgCl2 ✓½ - Starting with copper metal describe how a dry sample of copper II carbonate can be prepared in the laboratory. (3 marks)
- Add excess copper turnings to conc. Nitric V acid 1to then filter√ ½to obtain copper II nitrate solution as filtrate. To the filtrate add sodium carbonate √ ½ solutions to precipitate copper II carbonate √ ½. Filter, wash and dry the residue between filter papers. √ ½ 1
- The table below shows the first ionization energies of metals Ato D (not their actual chemical symbols) in the same group of the periodic table.
- Arrange the metals in order of that they occur in the periodic table starting from the topmost to the lowest. Give a reason to support your answer. (3marks)
- C, B, D, A 2 within a group of metals first ionization energy decreases down the group 1
- Which of the metals has the largest atomic radius? (1mark)
- A ✓½
- Arrange the metals in order of that they occur in the periodic table starting from the topmost to the lowest. Give a reason to support your answer. (3marks)
- When a piece of calcium is dropped into a beaker of water, it sinks to the bottom and bubbles of a gas are observed on the surface of the metal.
- Why does calcium sink to bottom of the beaker? (1mark)
- Its density is higher than that of water
- Name the gas that is formed in the reaction. (1mark)
- Hydrogen gas
- Besides effervescence, what else is observed in the beaker as the reaction progresses?Explain this observation. (2marks)
- White suspension, calcium hydroxide is slightly soluble in water
- Write an equation for the reaction between calcium and water. 1mk
Ca + 2H2O−Ca(OH)−2 + H2
- Why does calcium sink to bottom of the beaker? (1mark)
- Explain the following statements:
- Following a bee’s sting, application of sodium hydrogen carbonates to the affected area of the skin reliefs the irritation. (2marks)
- Bee sting contains methanoic acid (weak acid) which irritates✓1 , the acid is neutralized by sodium hydrogen carbonate.✓1
- It is not advisable to clean aluminium utensils using wood ash . (2marks)
- Aluminium utensils have a tough aluminium oxide coat on the surface ✓1 that protects the metal beneath, wood ash is alkaline and reacts with amphoteric Al2O3 coat removing it thus exposing the metal to corrosion.✓1
- Following a bee’s sting, application of sodium hydrogen carbonates to the affected area of the skin reliefs the irritation. (2marks)
- The set-up below was used to investigate some properties of hydrogen gas. Study it and answer the questions that follow:
- Name a suitable liquid that can serve as a drying agent. (1mark)
- Conc sulphuric acid
- State the observations you would expect in the combustion tube as the experiment progresses. (2mks)
- White anhydrous copper (ii) sulphate turns blue
- PbO (orange when hot yellow) when cold turns grey
- Colourless droplets forms at the cooler parts of the apparatus
- Name a suitable liquid that can serve as a drying agent. (1mark)
- Explain the following terms:
- Water of crystallization (1mark)
- Water of crystallization is a fixed amount of water that is contained in the crystal structure of most salts. ✓1
- Hygroscopy (1mark)
- hygroscopy is the taking up water vapour from the atmosphere by a salt.✓1
- Acidic salts
- Salts with a replaceable hydrogen ion
- Normal salts
- A salt that that does not have a replaceable hydrogen ion
- Water of crystallization (1mark)
- Explain the role of helium in the welding of metals. (2marks)
- Provide an inactive atmosphere which prevent oxidation of ho metal by air
- Whereas hydrogen was commonly used in airships and weather balloons earlier on it is no longer used nowadays. Give a reason for this. (1mark)
- Hydrogen gas is explosive
- Chebet, Mutua and Waweru are international athletes. Paper chromatography was used to test for the presence of illegal drugs in their blood which enhance the performance. The diagram below shows the chromatogram with the illegal drug labeled N.
- Who among them tested positive for the illegal drug? Explain. (2marks)
- L and K
- Explain what is meant by ‘solvent front’. (1mark)
- The furthest distance the solvent can reach
- Who among them tested positive for the illegal drug? Explain. (2marks)
- An aqueous solution of ammonia was added drop wise to a solution of copper (II) Sulphate until in excess
- State the observation made when
- A few drops of aqueous ammonia were added. (1 mark)
- A blue ppt is formed
- Excess aqueous ammonia was added. (1 mark)
- Blue ppt dissolves to a deep blue solution
- A few drops of aqueous ammonia were added. (1 mark)
- State the observation made when
- The table below gives information about the ions W+ and Y2−
- How many protons are there in the nucleus of
- Elements W? (1 mark)
- 11
- Elements Y? (1 mark)
- 16
- Elements W? (1 mark)
- Write the formula of the compound formed when W and Y reacts. (1 mark)
- W2Y
- State two conditions under which the compound would conduct electricity. (2marks)
- Molten state
- Aqueous state
- How many protons are there in the nucleus of
- The following data gives the PH vales of some solution A, B and C.
- Which solution would produce carbon (IV) oxide gas when reacted with copper (II) carbonate? Explain. (2 mark)
- C, it has hydrogen ions
- What colour change would occur in solution A on addition of three drops of phenolphthalein indicator. (1 mark)
- pink
- What volume of 0.2 M hydrochloric acid would react completely with 0.005 moles of pure calcium carbonate? (3 marks)
2HCl(aq) + CaCO3(aq)CaCl2(aq) + CO2(g) + H2O(l)
0.2M 0.005moles
Mole ratio 2 : 1
Moles of HCL = 0.005 × 2
= 0.01
1000cm3 = 0.2Moles
x = 0.01moles
x = 0.01 × 1000
0.2
- Which solution would produce carbon (IV) oxide gas when reacted with copper (II) carbonate? Explain. (2 mark)
- What is an allotrope? (1mark)
- A substance that occurs in more than one form but same physical state.
- Give two allotropes of sulphur. (2marks)
- Rhombic
- Monoclinic
- State three uses of sulphur. (3marks)
- Manufacture of sulphuric acid
- Hardening of rubber
- Manufacture breaching agent
- As a fungicide
- Give two allotropes of sulphur. (2marks)
- A substance that occurs in more than one form but same physical state.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Form 3 Term 2 Opener Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

