-
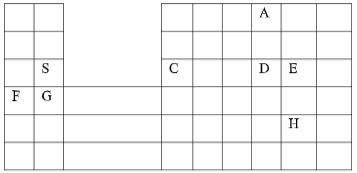
- Study the following part of periodic table chart and use it to answer the questions that follow. The letters are not the actual symbols of the elements.
- Which elements form ions with charge of -2? Explain (2mks)
- If the oxides of B and D are separately dissolved in water, what effect will their aqueous solution have on litmus. (2mks)
- How would you expect the ionic reactions of C and E to compare? Explain (2mks)
- Write the formula of the compounds formed between elements G and H (1mk)
- In terms of structure and bonding, explain why the oxide of D has a lower melting point than the oxides of B. (2mks)
- Write an equation to show the action of heat on the carbonates with element G (1mk)
- When 1.5litres of chlorine gas were completely reacted with element B 5.937g of the product were formed. Determine the relative atomic mass of element B. (Atomic mass of chlorine = 35.5, Molar gas volume = 24litres) (3mks)
- Study the following part of periodic table chart and use it to answer the questions that follow. The letters are not the actual symbols of the elements.
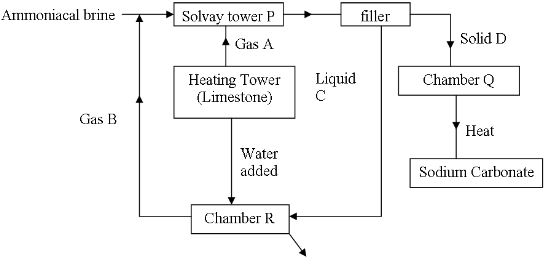
- The scheme below shows the manufacture of sodium carbonate by the Solvay process. Study it and use it to answer the questions that follow.
- Name
- Gases A and B (1mark)
- Name liquid C and Solid D (1mark)
- Write equations for the reactions taking place in tower P and chamber R (2marks)
- Name the product formed in chamber at chamber R and give one of its uses (2marks)
- State two uses of sodium carbonate (1mark)
- Name
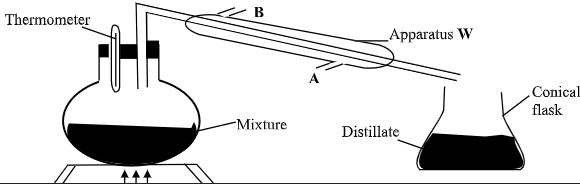
- A student left some crushed fruit mixture with water for some days. He found the mixture had fermented. He concluded that the mixture was contaminated with water and ethanol with boiling point of 100°C and 78°C respectively. The set-up of apparatus below are used to separate the mixture.
- Name the piece of apparatus labelled W (1mk)
- What is the purpose of the thermometer in the set-up? (1mk)
- At which end of the apparatus W should tap water be connected?(1mk)
- Which liquid was collected as the first distillate? Explain (2mk)
- What is the name given to the above method of separating mixture?(1mk)
- State two applications of the above method of separating mixtures (1mk)
- What properties of the mixture make it possible for the component to be separated by the above methods?(2mk)
-
- In an experiment, a piece of magnesium ribbon was cleaned with steel wool. 2.4g of the clean magnesium ribbon was placed in a crucible and completely burnt in oxygen. After cooling the product weighed 4.0g
- Explain why it is necessary to clean magnesium ribbon (1mks)
- What observation was made in the crucible after burning magnesium ribbon?(1mk)
- Why was there an increase in mass?(1mk)
- Write an equation for the major chemical reaction which took place in the crucible(1mk)
- The product in the crucible was shaken with water and filtered. State and explain the observation which was made when red and blue litmus paper were dropped into the filtrate (3mks)
- Below is a list of oxides.
MgO, N2O , K2O, CaO and Al2O3
Select:-- A neutral oxide. (1mk)
- A highly water soluble basic oxide. (1mk)
- An oxide which can react with both sodium hydroxide solution and dilute hydrochloric acid. (1mk)
- In an experiment, a piece of magnesium ribbon was cleaned with steel wool. 2.4g of the clean magnesium ribbon was placed in a crucible and completely burnt in oxygen. After cooling the product weighed 4.0g
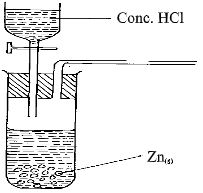
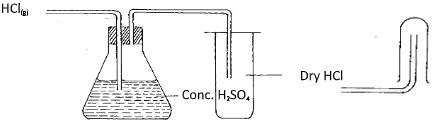
- The set-up below was used by a form three student to prepare a dry sample of gas M. Study it and use it to answer the questions that follow:-
- Complete the diagram to show how a dry sample of gas M can be collected (3mks)
- State the identity of gas M (1mk)
- state two industrial uses of gas M.(2mks)
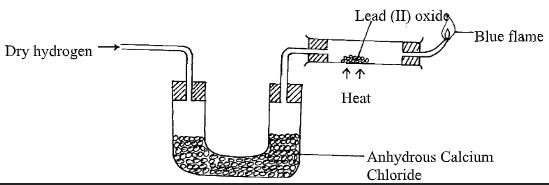
- What property of concentrated sulphuric acid is being employed in the above preparation? (1mk) The set-up below was used to investigate the properties of hydrogen.
- State the observations that was made in the combustion tube as the reaction progressed to completion (2mks)
- Write equations for the reactions ;
- In the combustion tube (1mk)
- At the jet of the delivery tube (1mk)
-
- Naturally occurring boron exists as two isotopes, boron-10 B with a relative abundance of 20% and boron-11 B with a relative abundance of 80%.
- How many electrons does each atom of boron contain? (1mk)
- How many neutrons does each atom of the most abundant isotope contain? (1mk)
- Calculate the relative atomic mass of boron. (2mks)
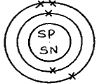
- Make a diagrammatic representation of an atom of the least abundant isotope of boron showing the distribution of electrons and composition of the nucleus. (2mks)
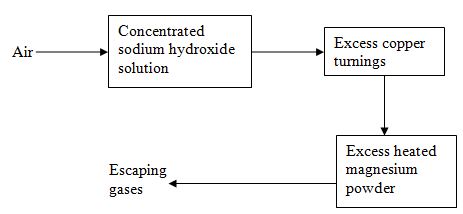
- Air was passed through several reagents as shown below:
- Write an equation for the reaction which takes place in the chamber containing
Magnesium powder (1mk) - Name one gas which escapes from the chamber containing magnesium powder.
Give a reason for your answer (1mk) - State two industrial uses of hydrogen gas (1mk)
- Write an equation for the reaction which takes place in the chamber containing
- Naturally occurring boron exists as two isotopes, boron-10 B with a relative abundance of 20% and boron-11 B with a relative abundance of 80%.
- The set-up below was used by a form three student to prepare a dry sample of gas M. Study it and use it to answer the questions that follow:-
- In the preparation of magnesium carbonate, magnesium was burnt in air and the product collected. Dilute sulphuric acid was then added and the mixture filtered and cooled. Sodium carbonate was added to the filtrate and the contents filtered. The residue was then washed and dried to give a white powder.
- Give the name of the product (1mk)
- Write the chemical equation for the formation of the product (1mk)
-
- Name the filtrate collected after sodium carbonate was added.(1mk)
- Write down the chemical formula of the white powder (1mk)
- Write a chemical equation for the reaction between product in (a) and the acid (1mk)
- Write an ionic equation to show the formation of the white powder(1mk).
- Write an equation to show what happens when the white powder is strongly heated. (1mk)
- Identify the ions present in the filtrate after addition of sodium carbonate. (1mk)
- What is the name given to the reaction that takes place when sodium carbonate was added to the filtrate? (1mkss)
-
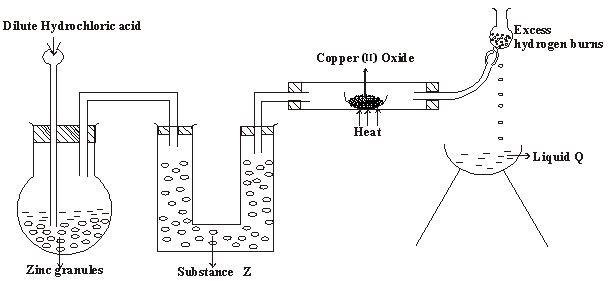
- In an experiment to investigate the properties of hydrogen, a student set up as follows.
- Name substances (2 marks)
Z
Q - State two properties of hydrogen that were being investigated. (2marks)
- Give two precautions that should be taken towards the end of the experiment. (2marks)
- State two reasons why it is not suitable to use dilute nitric (V) acid in the preparation of hydrogen with zinc. (2marks)
- Name substances (2 marks)
- Water molecule (H2O) combines with H + to form hydroxonium ion. (H3O+ ) explain. (2marks)
- Give TWO reason why hydrogen is used to fill meteorological balloons. (2mark)
- In an experiment to investigate the properties of hydrogen, a student set up as follows.
MARKING SCHEME
-
-
- Element A and B
Both have 6 electrons to achieve an octet. - Oxide of B forms an alkaline solution that turns red litmus blue.
Oxide of D forms acidic solution, that turns blue litmus red. - E has a bigger ionic radius than the ionic radius of C.
E forms ions / ionizes by gaining electrons; which C ionizes by lose of electrons. - Formula; GH2 (Rej H2G)
- Oxide of D is molecular with weaker vander waals forces, while the oxide of B is a giant ionic structure with stronger ionic bonds.
- GCO3(S) → GO(S) +CO2(g)
- Element A and B
- B + Cl2 → BCl2
1.5 litres of Cl2 → 5.9375 of BCl2
24
24 litres of Cl2 = (5.9375 × 1.5)g BCL2
= 95g
RFM of BCl2 = 95
RAM of BCl2 = 95 – 71=24
Or
B + Cl2 → BCl2
1.5
Moles of Cl2 used = 24 = 0.0625 moles
0.0625 moles Cl2 = 5.9375g BCl2
(5.9377g)
0.0625
1 mole = 95g 0g BCl2
RAM of B = 95 – 71 =24.
-
-
-
- Gas A – Carbon (iv) oxide
- Gas B – Ammonia gas ½mk
- Liquid C – Ammonium Chloride Solution ½mk
Solid D - Sodium Hydrogen Carbonate ½mk - NH4 HCO3 (aq) + NaCl (aq) → NaHCO3 (s) + NH4Cl (aq)
Ca (OH)2(aq) + 2NH4Cl (aq) → CaCl2 (aq) ++ 2NH3 (g) + 2H2O (l)
Penalize ½mk if not balance
½mk if there are no states - Ammonia
- Manufacture of fertilizers
- Manufacture of Nitric acid
- Refrigerant
- Softening water
- CaCl2 - Drying agent Name 1mk
- Use
- Making of glass
- Softening water
- Making sodium silicate used in making detergents
- Paper Industry
any two 1mk
-
- Condenser
- To indicate when a liquid is boiling, a thermometer reads a constant temperature
- A
- Ethanol
Reason:- It has a lower boiling of 78°C compared to water with a boiling point of 100°C or
The liquid with the lower boiling point boils first and its vapours are condensed and the condenser to be collected as the first distillate - Fractional distillation
-
- To separate components of crude oil
- To isolate O 2 and N 2 from air
- To manufacture spirits
- They are immiscible liquids
- They have different but close boiling points
-
-
- To remove any magnesium oxide coating from the surface of magnesium// To remove any oxide film on it
- White solid which is magnesium oxide
- Increase in mass was due to oxygen which combined with magnesium
- 2Mg(s) + O2(g) _______ 2MgO(s)
Penalize ½mk for wrong or missing state symbols - The filtrate is magnesium hydroxide which is an alkaline
Red litmus paper changed blue, but blue litmus paper remained blue
-
- N2O1 (Nitrogen (I) oxide) – Denitrogen Oxide.
- K2O1 (Potassium oxide)
- Al2O3 (Aluminium oxide)
- Yellow lead (II) oxide turned to red then grey.
-
- H2(g) + PbO(s) H2O(l) + Pb(s)
- 2H2(g) + O2(g) 2H2O (l)
- Reducing properties of hydrogen
Combustion nature of hydrogen
-
-
- M is hydrogen
- Conc. H2SO4 is a less volatile hence displaces a more volatile and from its salts i.e
-
- 5 electrons
- 11 – 5 = 6 neutrons
- 20/100 x 10 + 80/100 x 11 = 2+ 8.8 = 10.8
-
- 3Mg + N2g → Mg3N2g
- Argon
- It is inert
- It is inert
- Haber process to manufacture ammonia
Hydrogenation
Welding
-
-
- magnesium Oxide
- 2Mg(s) + O2(g )→ 2MgO(s)
-
- Sodium sulphate
- MgCO3
- MgO(s) + H2SO4(aq) → MgSO4(aq) + H2O(L)
- Mg2+ (aq) + CO2-3(aq) →M g CO 3(s)
- MgCO3(g) → MgO (g) + CO2(g)
- Na + ions and SO42- ions
- Precipitation/ double decomposition
-
-
- Z - Anhydrous calcium chloride 1mk
Q - Water - Reducing agent / effect 1mk
Combustible gases / burning of hydrogen in air. - The flame should be blown out ½ mk first as the supply of hydrogen continues to avoid explosion. ½mk Heating of CuO should be ½ mk stopped to prevent re-oxidation ½ mk of hot copper before ½ mk the supply of hydrogen is stopped.
- Hydrogen so produced is at once oxidized to water 1mk ( strong oxidizing agent )
Likelyhood of producing poisonous gases such as nitrogen (IV) oxide. √1mk
- Z - Anhydrous calcium chloride 1mk
- Water molecules has lone pairs 1mk of electrons which can be donated √mk and be shared with H+ to form H3O+
- Is less dense than air / lighter than air. 1mk
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - End Term 2 Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students