CHEMISTRY
PAPER 1
(THEORY)
FORM 4 TERM 1 OPENER EXAMS
TIME: 2 HOURS
INSTRUCTIONS TO CANDIDATES
- Answer ALL the questions in the paper
- Mathematical tables and silent electronic calculators may be used.
- All questions should be answered in English.
-
- What role do the following parts play during fractional distillation of water and ethanol?
- The fractionating column. (1mark)
- The glass beads (1mark)
- State one application of fractional distillation. (1mark)
- What role do the following parts play during fractional distillation of water and ethanol?
- Study the table below and answer the questions that follow:-
Ion Electron Arrangement R2+ 2.8.8 S2- 2.8 - Write the electron arrangement of each atom.
R _______ (½ mark)
S _______ (½ mark) - Write the formula of the oxide of R and Chloride of S
Oxide of R (1mark)
Chloride of S (1mark)
- Write the electron arrangement of each atom.
- When 5.35g of Sodium Nitrate were heated in an open crucible, the mass of oxygen produced was 0.83g. given that the equation for the reaction is:-
2NaNO3(s) → 2NaNO2(s) + O2(g)
Calculate the percentage of Sodium Nitrate that was converted to sodium nitrite. (3marks)
(Na=23, O=16, N = 14) - Equal volumes of water put in 100cm3 glass beaker and heated for 5 minutes using Bunsen flames. It was observed that water in beaker A registered higher temperature than beaker B.
- Name the kind of flame used in beaker; A (1mark)
- State the condition under which flame that heated B was produced. (1mark)
- Silver chloride can be prepared in the laboratory by the reaction between potassium chloride and silver nitrate.
- What name is given to this method of reaction? (1mark)
- Write an ionic equation for the reaction that occurs. (1mark)
- During heating of hydrated copper (II) sulphate crystals, the following readings were obtained:-
Mass of evaporating dish = 300g
Mass of evaporating dish + hydrated salt = 305g
Mass of evaporating dish + dehydrated salt = 303.2g
Calculate the empirical formula of hydrated copper (II) sulphate.
(Cu = 64.5, S = 32.0, O= 16.0, H = 1) (3marks) - A seed catalogue that the preferred soil pH range for the growth of different varieties of a crop are as shown in the table.
Type of plant Preferred pH A 4.5 – 6.0 B 5.0 – 7.5 C 5.5 – 6.5 D 6.0 – 6.5 - Which seed variety will grow over the largest pH range. (1mark)
- What soil pH range will a gardener be able to grow all these crops (1mark)
- The soil in a garden has a pH of 4.5, which substance can be added to the soil in order to grow plant type D? explain (2marks)
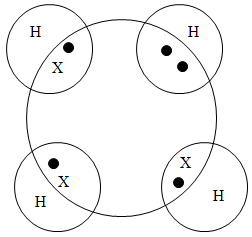
- Using dot (.) and cross (x) diagram, show bonding in the compound Ammonium ion (NH+4).(N= 14, H = 1) (2marks)
-
- State Graham’s Law of diffusion. (1mark)
- 200cm3 of oxygen gas take 250seconds to diffuse through a porous plug. Under similar conditions, an equal volume of an unknown gas take 277 seconds to diffuse through the same porous plug. Calculate the relative molecular mass of the unknown gas. (3marks)
- Give two products formed when a candle burns. (1mark)
- From the above products; which elements make up a candle? (1mark)
- Explain how the following substances conduct an electric current.
- Magnesium metal. (1mark)
- Molten magnesium chloride (1mark)
- The molar mass of a gaseous compound XO2 is 64 gmol-1. A sample of this gas occupied 11.2dm3 at s.t.p.molar gas volume =22.4dm3.find
- The number of moles of this gas. (2marks)
- The amount in grams that occupied the above volume. (2marks)
- Classify the following processes as either chemical or physical. (3marks)
Heating copper (II) sulphate crystals . Obtaining kerosene from crude oil . Souring of milk . - The diagram below represents a charcoal burner. Study it and answer the questions that follow;
Write equations for the reactions taking place in part I and II.
I (1mark)
II (1mark) - When a mixture of iron filings and sulphur are heated, a red glow spreads through the mixture and a dark grey solid was formed.
- Identify the dark grey solid formed. (1mark)
- Write a chemical equation in which the dark grey solid is formed. (1mark)
- What observation can be made when dark grey solid reacts with dilute hydrochloric acid. (1mark)
- The table below shows isotopes and their percentage abundances.
Calculate the relative atomic mass of the element with the above isotopes. (3marks)Isotope A B C Isotope mass 54 56 57 Percentage abundances 6.0 92.0 2.0 - Metal R does not react with an oxide of metal S. Metal T reacts with an oxide of metal S. metal Q reacts with an oxide of T. Arrange the metals in increasing order of reactivity. (2marks)
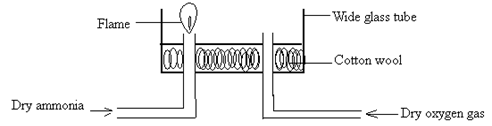
- An experiment was set up as shown below.
- A student passed dry ammonia gas into the glass tube and tried to ignite the gas before allowing oxygen gas. What observation was made? (1mark)
- Write the equation for the reaction above (1mark)
-
- Complete the table below to show pairs of substances used to prepare oxygen. (2marks)
Hydrogen peroxide Sodium peroxide - State the importance of oxygen in cutting metals. (1mark)
- Complete the table below to show pairs of substances used to prepare oxygen. (2marks)
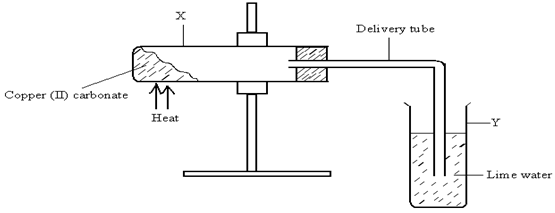
- The diagram below illustrates an experiment set up to investigate the effect of heat on copper (II) carbonate. Study it and answer the questions that follow.
- Give the expected observation in each test tube.
X ______(1mark)
Y ______(1mark) - Write an equation for the change that occurs in tube X (1mark)
- Give the expected observation in each test tube.
-
- State one way in which the strength of a base or an acid can be determined in the laboratory. (1mark)
- Give the basicity of the following acids:-
- Sulphuric (VI) acid. (1mark)
- Phosphoric acid (1mark)
- An element X is represented as 1840X (X = is not the actual symbol of the element)
- What is the composition of the nucleus for this element? (2marks
- Give the electronic arrangement of the element. (1mark)
- Chlorine gas was bubbled through a solution of potassium iodide in boiling tube.
- State the observation made. (1mark)
- Name the oxidizing agent in this reaction. Explain. (2marks)
- Name the process which take place when:-
- Iodine changes directly from solid to gas (1mark)
- Fe2+ changes to Fe3+ (1mark)
- White sugar changes to black solid when mixed with excess concentrated sulphuric acid. (1mark)
- GSTRU and P belong to the same period in the periodic table. The ions formed by the atoms are as below:-
Q2+, U-, T2-, R3+, P+, S3-- Arrange the elements in order of decreasing atomic size. (1mark)
- Suggest a reason why elements P and Q cannot react with each to form a compound. (1mark)
- A piece of burning magnesium ribbon was plunged into a gas jar containing sulphur (IV) oxide.
- What observation was made? (1mark)
- Write an equation for the reaction taking place. (1mark)
- What property of sulphur (IV) oxide is investigated above. (1mark)
- What is paper chromatography? (1mark)
- Give two applications of chromatography? (2marks)
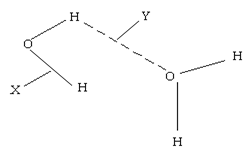
- The structure of two molecules of water can be represented as shown below.
Name the type of bonds X and Y (2marks)
X __________
Y __________ - In an experiment 3.36g of iron fillings were added to excess copper (II) sulphate. Calculate the mass of copper that was deposited. (CU= 63.5, Fe = 56) (3mks )
MARKING SCHEME
-
- What role do the following parts play during fractional distillation of water and ethanol?
- The fractionating column. (1mark)
- Fractionating column; allow water vapour to condense into liquid and flow back to the flask before boiling point of water is reached. √1mk
- Fractionating column; allow water vapour to condense into liquid and flow back to the flask before boiling point of water is reached. √1mk
- The glass beads (1mark)
- Glass beads; - increase the surface area for condensation of water to take place. √1
- Glass beads; - increase the surface area for condensation of water to take place. √1
- The fractionating column. (1mark)
- State one application of fractional distillation. (1mark)
- Distillation of liquid Air in the manufacture of nitrogen and oxygen √1mk or
- Distillation of crude oil.
- What role do the following parts play during fractional distillation of water and ethanol?
- Study the table below and answer the questions that follow:-
Ion Electron Arrangement R2+ 2.8.8 S2- 2.8 - Write the electron arrangement of each atom.
R - 2.8.8.2 (½ mark)
S - 2.6 (½ mark) - Write the formula of the oxide of R and Chloride of S
- Oxide of R (1mark)
- RO
- Chloride of S (1mark)
- SCl2
- SCl2
- Oxide of R (1mark)
- Write the electron arrangement of each atom.
- When 5.35g of Sodium Nitrate were heated in an open crucible, the mass of oxygen produced was 0.83g. given that the equation for the reaction is:-
2NaNO3(s) → 2NaNO2(s) + O2(g)
Calculate the percentage of Sodium Nitrate that was converted to sodium nitrite. (3marks)
(Na=23, O=16, N = 14)- Moles of oxygen gas. = 0.83/32
= 0.02594 √1mk
Moles of 2NaNO3 : O2
2 : 1
Moles of NaNO3 = 2 x 0.02594.
= 0.05188 moles √ ½ mk
Mass of NaNO3 = 85 x 0.05188
= 4.4098g √ ½ mk
Percentage of NaNO3 = 4.098/5.35 X 4.098/5.35 x 100 = 82.45%
- Moles of oxygen gas. = 0.83/32
- Equal volumes of water put in 100cm3 glass beaker and heated for 5 minutes using Bunsen flames. It was observed that water in beaker A registered higher temperature than beaker B.
- Name the kind of flame used in beaker; A (1mark)
- A = Non Luminous
- A = Non Luminous
- State the condition under which flame that heated B was produced. (1mark)
- When the air hole is closed. √1mk
- When the air hole is closed. √1mk
- Name the kind of flame used in beaker; A (1mark)
- Silver chloride can be prepared in the laboratory by the reaction between potassium chloride and silver nitrate.
- What name is given to this method of reaction? (1mark)
- Double decomposition (precipitation )
- Double decomposition (precipitation )
- Write an ionic equation for the reaction that occurs. (1mark)
- Ag+(aq) + Cl-(aq) → AgCl(s)
- Ag+(aq) + Cl-(aq) → AgCl(s)
- What name is given to this method of reaction? (1mark)
- During heating of hydrated copper (II) sulphate crystals, the following readings were obtained:-
Mass of evaporating dish = 300g
Mass of evaporating dish + hydrated salt = 305g
Mass of evaporating dish + dehydrated salt = 303.2g
Calculate the empirical formula of hydrated copper (II) sulphate.
(Cu = 64.5, S = 32.0, O= 16.0, H = 1) (3marks)- Empirical formula
Compounds present : CuSO4 : nH2O
Mass present 3.2 : 1.8
R.F.M 160.5 : 18√1mk
No of moles 3.2/160.5 : 1.8/18√ ½ mk
0.02 : 0.1
Mole ratio 0.02/0.02 : 0.1/0.02√ ½ mk
1 : 5
E.F. CuSO4 .5H2O √1mk
- Empirical formula
- A seed catalogue that the preferred soil pH range for the growth of different varieties of a crop are as shown in the table.
Type of plant Preferred pH A 4.5 – 6.0 B 5.0 – 7.5 C 5.5 – 6.5 D 6.0 – 6.5 - Which seed variety will grow over the largest pH range. (1mark)
- Variety B
- Variety B
- What soil pH range will a gardener be able to grow all these crops (1mark)
- 5.5-6.5 Soil Ph or 5.0-7.5
- 5.5-6.5 Soil Ph or 5.0-7.5
- The soil in a garden has a pH of 4.5, which substance can be added to the soil in order to grow plant type D? explain (2marks)
- Add lime water which is basic for the soil PH to be neutral√1mk
- Add lime water which is basic for the soil PH to be neutral√1mk
- Which seed variety will grow over the largest pH range. (1mark)
- Using dot (.) and cross (x) diagram, show bonding in the compound Ammonium ion (NH+4).(N= 14, H = 1) (2marks)
-
- State Graham’s Law of diffusion. (1mark)
- The volume of a fixed mass of a gas is inversely proportional to the square root of the density.
- The volume of a fixed mass of a gas is inversely proportional to the square root of the density.
- 200cm3 of oxygen gas take 250seconds to diffuse through a porous plug. Under similar conditions, an equal volume of an unknown gas take 277 seconds to diffuse through the same porous plug. Calculate the relative molecular mass of the unknown gas. (3marks)
- 250/279 = √(32/mx)
250 x Jmx = 277 x √32
√mx= (277 x 532)/250
Mx = ((277 x √32)/250)
Mx = 39.29
- 250/279 = √(32/mx)
- Give two products formed when a candle burns. (1mark)
- Carbon (iv) oxide √1mk and water √1mk
- Carbon (iv) oxide √1mk and water √1mk
- From the above products; which elements make up a candle? (1mark)
- Carbon √1mk and hydrogen √1mk
- Carbon √1mk and hydrogen √1mk
- State Graham’s Law of diffusion. (1mark)
- Explain how the following substances conduct an electric current.
- Magnesium metal. (1mark)
- delocalized electrons
- delocalized electrons
- Molten magnesium chloride (1mark)
- mobile ions
- mobile ions
- Magnesium metal. (1mark)
- The molar mass of a gaseous compound XO2 is 64 gmol-1. A sample of this gas occupied 11.2dm3 at s.t.p.molar gas volume =22.4dm3.find
- The number of moles of this gas. (2marks)
- I mole of a gas occupy - 22.4dm3 at s.t.p
? - 11.2dm3√1mk
(11.2 x 1)/22.4 = 0.5mol √1mk
- I mole of a gas occupy - 22.4dm3 at s.t.p
- The amount in grams that occupied the above volume. (2marks)
- 22.4dm3 → 64g/l
11.2dm3 → ?
11.2 x 64/22.4=32g √1mk
- 22.4dm3 → 64g/l
- The number of moles of this gas. (2marks)
- Classify the following processes as either chemical or physical. (3marks)
Heating copper (II) sulphate crystals reversing chemical change √1mk Obtaining kerosene from crude oil Physical change √1mk Souring of milk Permanent chemical change √1mk - The diagram below represents a charcoal burner. Study it and answer the questions that follow;
Write equations for the reactions taking place in part I and II.
I (1mark) - CO2(s) + C(s) → CO(g)
II (1mark) - 2CO(g) + O2(g) → CO(g) - When a mixture of iron filings and sulphur are heated, a red glow spreads through the mixture and a dark grey solid was formed.
- Identify the dark grey solid formed. (1mark)
- Iron (II) sulphide √1mk
- Iron (II) sulphide √1mk
- Write a chemical equation in which the dark grey solid is formed. (1mark)
- Fe(s) + S(s) → FeS √1mk
- Fe(s) + S(s) → FeS √1mk
- What observation can be made when dark grey solid reacts with dilute hydrochloric acid. (1mark)
- A gas with rotten egg smell is produced √1mk
- A pale green solution is formed
- Identify the dark grey solid formed. (1mark)
- The table below shows isotopes and their percentage abundances.
Calculate the relative atomic mass of the element with the above isotopes. (3marks)Isotope A B C Isotope mass 54 56 57 Percentage abundances 6.0 92.0 2.0 - R.A.M = 54 x 6+ 56 x 92 + 57 x 2
100 √1mk
= 324 + 5152 + 114
100 √1mk
= 5590/100
= 55.9√1mk
- R.A.M = 54 x 6+ 56 x 92 + 57 x 2
- Metal R does not react with an oxide of metal S. Metal T reacts with an oxide of metal S. metal Q reacts with an oxide of T. Arrange the metals in increasing order of reactivity. (2marks)
- RSTQ √1mk increasing reactivity √1mk
→
- RSTQ √1mk increasing reactivity √1mk
- An experiment was set up as shown below.
- A student passed dry ammonia gas into the glass tube and tried to ignite the gas before allowing oxygen gas. What observation was made? (1mark)
- Ammonia gas does not burn in air. Thus it did not ignite. √1mk
- Ammonia gas does not burn in air. Thus it did not ignite. √1mk
- Write the equation for the reaction above (1mark)
- The gas ignites with green – yellow flame √1mk
- 4NH3(g) + 3O2(g) → 2N2(g) + 6H2O(g) √1mk
- A student passed dry ammonia gas into the glass tube and tried to ignite the gas before allowing oxygen gas. What observation was made? (1mark)
-
- Complete the table below to show pairs of substances used to prepare oxygen. (2marks)
Hydrogen peroxide Manganese (IV) oxide √1mk Water √1mk Sodium peroxide - State the importance of oxygen in cutting metals. (1mark)
- Oxy – hydrogen √1mk very hot flames for cutting of metal
- Oxy – acetylene
- Complete the table below to show pairs of substances used to prepare oxygen. (2marks)
- The diagram below illustrates an experiment set up to investigate the effect of heat on copper (II) carbonate. Study it and answer the questions that follow.
- Give the expected observation in each test tube.
X - green copper (II) carbonate changes to black copper (ii) oxide.(1mark)
Y - The colorless solution of limewater turns to a white ppt. √1mk(1mark) - Write an equation for the change that occurs in tube X (1mark)
- CuCO3(s) → CuO(s) + CO2(g) √1mk
- CuCO3(s) → CuO(s) + CO2(g) √1mk
- Give the expected observation in each test tube.
-
- State one way in which the strength of a base or an acid can be determined in the laboratory. (1mark)
- By use of universal indicator solution √1mk and compairing the colour obtained with the PH scale.
- By use of universal indicator solution √1mk and compairing the colour obtained with the PH scale.
- Give the basicity of the following acids:-
- Sulphuric (VI) acid. (1mark)- 2
- Phosphoric acid (1mark) - 3
- State one way in which the strength of a base or an acid can be determined in the laboratory. (1mark)
- An element X is represented as 1840X (X = is not the actual symbol of the element)
- What is the composition of the nucleus for this element? (2marks)
- Protons 18 √1mk
neutrons 22√1mk
- Protons 18 √1mk
- Give the electronic arrangement of the element. (1mark)
- X : 2,8,8 √1mk
- X : 2,8,8 √1mk
- What is the composition of the nucleus for this element? (2marks)
- Chlorine gas was bubbled through a solution of potassium iodide in boiling tube.
- State the observation made. (1mark)
- Yellow colour of chlorine turns to colourless√1mk and a black solid is formed at the bottom of the solution.
- Yellow colour of chlorine turns to colourless√1mk and a black solid is formed at the bottom of the solution.
- Name the oxidizing agent in this reaction. Explain. (2marks)
- 2KI (aq) + Cl2(g) → 2KCl(aq) + I2(g)
- Chlorine is the oxidizing agent √1mk because its oxidation number changes form 0 to -1
- State the observation made. (1mark)
- Name the process which take place when:-
- Iodine changes directly from solid to gas (1mark)
- Sublimation √1mk
- Sublimation √1mk
- Fe2+ changes to Fe3+ (1mark)
- Oxidation √1mk
- Oxidation √1mk
- White sugar changes to black solid when mixed with excess concentrated sulphuric acid. (1mark)
- Dehydration √1mk
- Dehydration √1mk
- Iodine changes directly from solid to gas (1mark)
- GSTRU and P belong to the same period in the periodic table. The ions formed by the atoms are as below:-
Q2+, U-, T2-, R3+, P+, S3-- Arrange the elements in order of decreasing atomic size. (1mark)
- U ,T ,S ,R ,Q ,P √1mk
→
Decreasing atomic size
- U ,T ,S ,R ,Q ,P √1mk
- Suggest a reason why elements P and Q cannot react with each to form a compound. (1mark)
- Both P and Q need to loose √1mk electrons to become stable, therefore they cannot react to form a compound.
- Both P and Q need to loose √1mk electrons to become stable, therefore they cannot react to form a compound.
- Arrange the elements in order of decreasing atomic size. (1mark)
- A piece of burning magnesium ribbon was plunged into a gas jar containing sulphur (IV) oxide.
- What observation was made? (1mark)
- A yellow deposit of sulphur is observed and a white powder of MgO formed.
- A yellow deposit of sulphur is observed and a white powder of MgO formed.
- Write an equation for the reaction taking place. (1mark)
- 2Mg(s) + SO2(g) → 2MgO(s) + S(s) √1mk
- 2Mg(s) + SO2(g) → 2MgO(s) + S(s) √1mk
- What property of sulphur (IV) oxide is investigated above. (1mark)
- Oxidising agent/property
- Oxidising agent/property
- What observation was made? (1mark)
-
- What is paper chromatography? (1mark)
- A method used to separate coloured pigments. √1mk
- A method used to separate coloured pigments. √1mk
- Give two applications of chromatography? (2marks
- In food industry to identify contaminants in food and drinks. √1mk
- In sports to identify illegal substances e.g steroids in urine or blood samples. √1mk
- What is paper chromatography? (1mark)
- The structure of two molecules of water can be represented as shown below.
Name the type of bonds X and Y (2marks)
X - Covalent bond √1mk
Y - Hydrogen bond √1mk - In an experiment 3.36g of iron fillings were added to excess copper (II) sulphate. Calculate the mass of copper that was deposited. (CU= 63.5, Fe = 56) (3mks )
- Fe(s) + CuSO4(aq) → FeSO4(aq + Cu(s)
1mol 1mol
Moles of iron used = 3.36/56
= 0.06moles √1mk
Mole ratio of reaction
Fe : Cu
1 : 1√1
Moles of Cu produced is 0.06.
Thus mass of copper deposited
= 0.06 x 63.5
= 3.81g√1
- Fe(s) + CuSO4(aq) → FeSO4(aq + Cu(s)
Download Chemistry Paper 1 Questions and Answers - Form 4 Term 1 Opener Exams 2021.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students