CHEMISTRY
PAPER 1
Instructions
- Answer all questions
- Mathematical table and silent electronic calculators may be used.
- All working must be clearly shown where necessary.

Questions
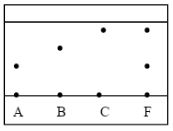
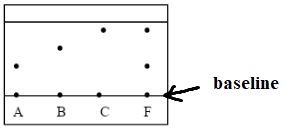
- Three pure pigments were prepared and their spots placed on a filter paper as shown below. The three pigments are A, B and C. A mixture F was also placed on the filter paper at the same time with the pure pigments. The filter paper was then dipped in ethanol solvent and left for some half an hour. The results were obtained as follows.
- Which of the three pure pigments is most sticky? Give a reason for your answer. (1mk)
- Which pure pigment is not present in the mixture F? (1mk)
- Show on the diagram the baseline. (1mk)
- Describe how a pure sample of lead (II) carbonate can be prepared in the laboratory starting with lead II oxide. (3mks)
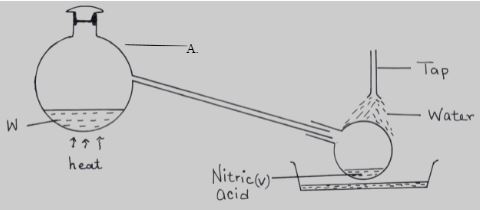
- The set up below was used to prepare nitric (V) acid in the laboratory.
- Name the mixture W. (1mk)
- Write an equation for the reaction that takes place in flask A. (1mk)
- Explain why concentrated nitric (V) acid produced appears yellow when exposed to sun light (1mk )
- A mixture contains ammonium chloride, aluminium oxide and sodium chloride. Describe how each solid substance can be obtained from the mixture. (3mks)
- State the difference between the following salts;
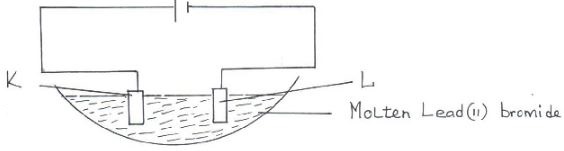
Deliquescent and hygroscopic salts. (2mks) - Below is a set-up of apparatus used to investigate the effect of electric current on molten lead (II) bromide.
- Name electrode K and L. (1mk)
- State the observation made at electrode K. (1mk)
- Write an equation for the reaction taking place at electrode L. (1mk)
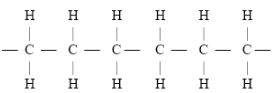
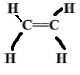
- A sample of a polyethene polymer has the following structure.
- Draw the structural formula of the monomer that makes the above polymer
- The polymer is found to have a molecular mass of 2268g. Determine the number of monomers in the polymer. (H = 1, C = 12). (2mks)
-
Study the information given in the table below and answer the questions that follows.
- Predict the cation and anion present, in solid H.
Cation (1mk)
Anion (1mk) -
Identify solid K, solution B and white-precipitate.
Solid K (1mk)
Solution B (1mk)
White precipitate(ppt) (1mk)
- Predict the cation and anion present, in solid H.
- The isotopes hydrogen are 11H and 21H. Determine the molecular masses of the molecules formed when each of these isotopes react with chlorine. (Cl = 37, H=1) (1mk)
- The table below gives the atomic numbers of elements W,X,Y and Z. The letters do not represent the . actual symbol of the elements
Element A B C D Atomic number 9 10 11 12 - Which one of the elements is unreactive? Explain (1mk)
-
- Which two elements would react most vigorously with each other? (1mk)
- Give the formula of the compound formed when the elements in b (i) above react (1mk)
-
- Distinguish between a hydrogen bond and covalent bond (2mks)
- Explain why the boiling point of water is higher than that of hydrogen Sulphide
(Relative molecular mass of water is 18 while that hydrogen sulphide is 34) (2mks)
- In an attempt to investigate the properties of halogens, a student bubbled chlorine gas through a solution of potassium bromide.
- State and explain what was observed. (2mks)
- Write an ionic equation for the reaction. (1mk)
- Explain why iodine sublimes when heated to form a purple vapour. (1mk)
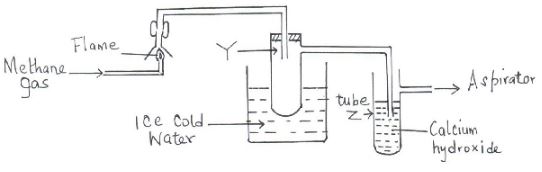
- The set-up below was used to investigate the products of burning methane gas. Study it and answer the questions that follow:
- What product will be formed in the test tube Y? (1mk)
- State and explain the observations made in tube Z. (2mks)
- Below are PH values of some solutions.
Solution Z Y X W pH 6.5 13.5 2.2 7.2 - Which solution is likely to be
- Acidic rain. (½mk)
- Potassium hydroxide (½mk)
- A basic substance V reacted with both solutions Y and X. What is the nature of V. (1mk)
- Which solution is likely to be
- In cold countries, salt is sprayed on the road to melt ice but in the long run it costs the motorists.
- How does the salt help in melting ice? (1mk)
- How does the salt affect the motorists? (1mk)
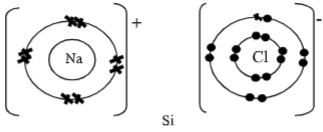
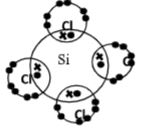
- Using dots (. ) and crosses (x) to represent electrons, show bonding in the compounds formed when the following elements react: (Si=14, Na=11, Cl=17).
- Sodium and chlorine. (2 Mks)
- Silicon and chlorine. (2 Mks)
-
- State Graham’s law of diffusion. (1mk)
- 20cm³ of an unknown gas Q takes 12.6 seconds to pass through a small orifice, 10cm³ of oxygen gas takes 11.2 seconds to diffuse through the same orifice under the same conditions of temperatures and pressure. Calculate the molecular mass of unknown gas Q (O = 16). (3mks)
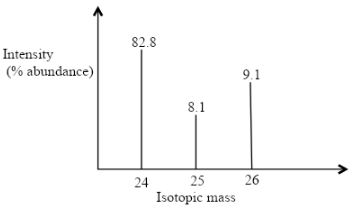
- The peaks below show the mass spectrum of element X.
Calculate the relative atomic mass of X. (2mks) - Name the following compounds using the IUPAC rules.
- CH3CH2CHCH2CH2CH3
|
CH2CH3
______________________________________________ (1mk) - CH3CHCHCH3 ___________________________________________ (1mk)
- Draw TWO structural formulae of isomers of compound with the molecular formula CH3CH2CH2CH3 (2mks)
- CH3CH2CHCH2CH2CH3
-
- What is meant by allotropy? (1 mk)
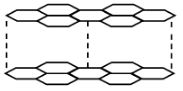
- The diagram below shows the structure of one allotropes of carbon.
- Identify the allotrope ( 1 mk)
- State one property of the above allotrope and explain how it is related to its structure. (2mk) .
- 24cm³ of a solution of 0.1 M potassium hydroxide were exactly neutralized by 30cm³ of a solution of sulphuric acid. Find the molarity of the acid. (3 mks)
-
- Give one use of hygroscopic substances in the laboratory. (1 mk)
- What is meant by the terms: (2 mks)
- Isotopes
- Mass number
- The formulae for a chloride of phosphorus is PCl3. What is the formula of its sulphide? (1 mk)
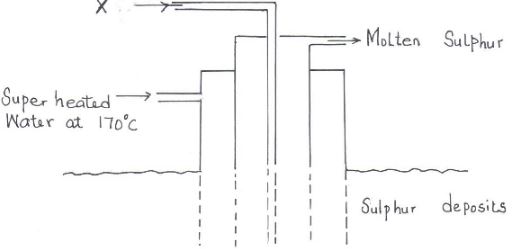
- The diagram below shows the Frasch process used for extraction of sulphur. Use it to answer the questions that follow.
- Identify X. (1mk)
- Why is it necessary to use super heated water in this process? (1mk)
- State two physical properties of sulphur that makes it possible for it to be extracted by this method. (1mk)
- A certain carbonate XCO3 , reacts with dilute hydrochloric acid according to the equation given below:
XCO3(s) +2HCl (aq) → XCl2(aq) + CO2(g) + H2O(l)
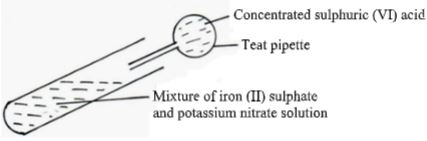
If 4g of the carbonate reacts completely with 40cm3 of 2M hydrochloric acid, calculate the relative atomic mass of X. (C=12.0 ,O=16.0, Cl=35.5). (3 Mks) - Concentrated sulphuric acid is slowly added to a mixture of freshly prepared solution of iron (II) sulphate and potassium nitrate as below.
- State the observation made. (1mk)
- The table below gives some properties of three substances I, J and K. Study it and answer the questions that follow.
Substance Mpt(°C) Solubility in water Electrical conductivity Solid Molten I 1063 Insoluble Conduct Conduct J 113 Insoluble Doesn't Doesn't K 402 Sparingly soluble Doesn't Conduct and
is decomposed- Suggest the type of structure in
- I (1mk)
- K (1mk)
- Explain why the molten K is decomposed by electric current but I is not decomposed.(2mks)
- Suggest the type of structure in

Marking Scheme
- Three pure pigments were prepared and their spots placed on a filter paper as shown below. The three pigments are A, B and C. A mixture F was also placed on the filter paper at the same time with the pure pigments. The filter paper was then dipped in ethanol solvent and left for some half an hour. The results were obtained as follows.
- Which of the three pure pigments is most sticky? Give a reason for your answer. (1mk)
- A – moves shortest distance
- A – moves shortest distance
- Which pure pigment is not present in the mixture F? (1mk)
- B
- B
- Show on the diagram the baseline. (1mk)
- Which of the three pure pigments is most sticky? Give a reason for your answer. (1mk)
- Describe how a pure sample of lead (II) carbonate can be prepared in the laboratory starting with lead II oxide. (3mks)
- Measure a fixed volume of nitric V acid in a beaker
- Add lead II oxide to the acid until in excess
- Filter the mixture and collect the filtrate lead II nitrate
- To the filtrate add a solution of sodium carbonate solution to precipitate lead carbonate
- Filter the mixture and collect the residue lead II carbonate wash the residue and dry it under low temperature or btw filter papers
- The set up below was used to prepare nitric (V) acid in the laboratory.
- Name the mixture W. (1mk)
- Conc. sulphuric (VI) acid + potassium nitrate
- Conc. sulphuric (VI) acid + potassium nitrate
- Write an equation for the reaction that takes place in flask A. (1mk)
- H2SO4(l) + KNO3(S) → KHSO4(aq) + HNO3(aq)
- H2SO4(l) + KNO3(S) → KHSO4(aq) + HNO3(aq)
- Explain why concentrated nitric (V) acid produced appears yellow when exposed to sun light (1mk )
- Nitric (V) acid decomposes on exposure to light to form nitrogen (IV) oxide. (1mk)
- Nitric (V) acid decomposes on exposure to light to form nitrogen (IV) oxide. (1mk)
- Name the mixture W. (1mk)
- A mixture contains ammonium chloride, aluminium oxide and sodium chloride. Describe how each solid substance can be obtained from the mixture. (3mks)
- Heat the mixture ammonium chloride sublimes and is collected on the cooler part of boiling tube./accept a diagram
- Add water to dissolve NaCl.
- Filter to obtain Al2O3 as a residue.
- Evaporate the filtrate to obtain NaCl (3mks)
- State the difference between the following salts;
- Deliquescent and hygroscopic salts. (2mks)
- Deliquescent – Absorbs water from atmosphere to form a solution.
- Hygroscopic – Absorbs water from the atmosphere and do not form solution. (2mks)
- Below is a set-up of apparatus used to investigate the effect of electric current on molten lead (II) bromide.
- Name electrode. (1mk)
- K Anode
- L Cathode
- State the observation made at electrode K. (1mk)
- Red-brown vapour
- Red-brown vapour
- Write an equation for the reaction taking place at electrode L. (1mk)
- Pb2+(l) + 2e- → Pb(s)
- Pb2+(l) + 2e- → Pb(s)
- Name electrode. (1mk)
- A sample of a polymer has the following structure.
- Draw the structural formula of the monomer that makes the above polymer
- Mass of monomer
- Mass of monomer
- The polymer is found to have a molecular mass of 2268g. Determine the number of monomers in the polymer. (H = 1., C = 12). (1mk)
- 2268/28 = 81 monomers
- 2268/28 = 81 monomers
- Draw the structural formula of the monomer that makes the above polymer
- Study the information given in the table below and answer the questions that follows.
- Predict the cation and anion present, in solid H.
Cation - Zn2+ (1mk)
Anion - NO-3 (1mk) - Identify solid K, solution B and white-precipitate.
Solid K - ZnO (1mk)
Solution B - ZnSO4 (1mk)
White precipitate(ppt) - Zn(OH)2(S)(1mk)
- Predict the cation and anion present, in solid H.
- The isotopes hydrogen are 11H and 21H. Determine the molecular masses of the molecules formed when each of these isotopes react with chlorine. (Cl = 37) (2mks)
- Molecule: HCl
MM. of 11HCl = 38
Of 21HCl = 39
- Molecule: HCl
- The table below gives the atomic numbers of elements W,X,Y and Z. The letters do not represent the . actual symbol of the elements
- Which one of the elements is un reactive? Explain (1mk)
- B has completely filled outer energy level
-
- Which two elements would react most vigorously with each other? (1mk)
- A and C
- Give the formula of the compound formed when the elements in b (i) above react (1mk)
- CA //NaF reject AC
- Which two elements would react most vigorously with each other? (1mk)
- Which one of the elements is un reactive? Explain (1mk)
-
- Distinguish between a hydrogen bond and dative covalent bond (2mks)
- hydrogen bond is formed between a hydrogen atom of one molecule with a more electronegative/an oxygen of another element of another molecule. (accept illustration e.g
- Covalent bond is formed when two electronegative elements bond by each donating an electron to be shared in the bond.
- Explain why the boiling point of water is higher than that of hydrogen Sulphide
- (Relative molecular mass of water is 18 while that hydrogen sulphide is 34) (2mks)
- Water has hydrogen bonding in addition to vanderwaals forces which makes the intermolecular force strong requiring more energy, while hydrogen sulphide has only weak vander waals forces which requires less energy to break.
- Distinguish between a hydrogen bond and dative covalent bond (2mks)
- In an attempt to investigate the properties of halogens, a student bubbled chlorine gas through a solution of potassium bromide.
- State and explain what was observed. (2mks)
- Brown/Orange solution formed. Chlorine displaces bromine from its solution.
- Brown/Orange solution formed. Chlorine displaces bromine from its solution.
- Write an ionic equation for the reaction. (1mk)
- 2Br-(aq) + Cl2(g) → 2Cl-(aq) + Br2(g)
- 2Br-(aq) + Cl2(g) → 2Cl-(aq) + Br2(g)
- Explain why iodine sublimes when heated to form a purple vapour. (1mk)
- Iodine molecules are joined together by weak intermolecular forces of
- Attraction/weak van der waal forces that are easily broken on heating. (1mk)
- State and explain what was observed. (2mks)
- The set-up below was used to investigate the products of burning methane gas. Study it and answer the questions that follow:
- What product will be formed in the test tube Y? (1mk)
- Water/H2O
- Water/H2O
- State and explain the observations made in tube Z. (2mks)
- A white precipitate. ✓½ Burning methane produces carbon (IV) oxide ✓½
- Which reacts with calcium hydroxide to form the insoluble ✓½ calcium carbonate ✓½
- What product will be formed in the test tube Y? (1mk)
- Below are pH values of some solutions.
- Which solution is likely to be
- Acidic rain. - Z
- Potassium hydroxide-Y (1mk)
- A basic substance V reacted with both solutions Y and X. What is the nature of V. (1mk)
- Amphoteric
- Amphoteric
- Which solution is likely to be
- In cold countries, salt is sprayed on the road to melt ice but in the long run it costs the motorists.
- How does the salt help in melting ice? (1mk)
- Salt acts as an impurity. Lowers Mpt of ice hence ice melts at low Mpt.
- Salt acts as an impurity. Lowers Mpt of ice hence ice melts at low Mpt.
- How does the salt affect the motorists?
- Salt increases rate of rusting of the metallic parts of the vehicles. (1mk)
- Salt increases rate of rusting of the metallic parts of the vehicles. (1mk)
- How does the salt help in melting ice? (1mk)
- Using dots ( ) and crosses (x) to represent electrons, show bonding in the compounds formed when the following elements react: (Si=14, Na=11, Cl=17).
- Sodium and chlorine. (2 Mks)
- Silicon and chlorine. (2 Mks)
- Sodium and chlorine. (2 Mks)
-
- Define Graham’s law of diffusion.
- Under the same conditions of temperature and pressure, the rate of diffusion of a gas is inversely proportion to the square root of its density. (1mk)
- Under the same conditions of temperature and pressure, the rate of diffusion of a gas is inversely proportion to the square root of its density. (1mk)
- 20cm³ of an unknown gas Q takes 12.6 seconds to pass through small orifice, 10cm³ of oxygen gas takes 11.2 seconds to diffuse through the same orifice under the same conditions of temperatures and pressure. Calculate the molecular mass of unknown gas Q (O = 16). (3mks)
- TQ/MO₂ = √(MQ/MO₂)
TQ = 12.6 sec
TO2 = 22.4 sec
MO2= 2 x 16=32
12.6/22.4 = √(MQ/32)
MQ = (12.6/22.4)2 x 32
=10.13
- TQ/MO₂ = √(MQ/MO₂)
- Define Graham’s law of diffusion.
- The peaks below show the mass spectrum of element X.
Calculate the relative atomic mass of X. (2mks)- (24 x 82.8) + (25 x 81) + (26 x 9.1)
100
1987.2 + 202.5 + 236.6
100
2426.3
100
= 24.263
- (24 x 82.8) + (25 x 81) + (26 x 9.1)
- Name the following compounds using the IUPAC rules.
- CH3CH2CHCH2CH2CH3
|
CH2CH3- 3-ethyl hexane (1mk)
- 3-ethyl hexane (1mk)
- CH3CHCHCH3
- But-2-ene (1mk)
- But-2-ene (1mk)
- Draw TWO structural formulae of isomers of compound with the molecular formula CH3CH2CH2CH3 (2mks)
- CH3CH2CHCH2CH2CH3
-
- What is meant by allotropy? (1 Mk)
- allotropy is the existence of an element in more than one form without change in physical state
- allotropy is the existence of an element in more than one form without change in physical state
- The diagram below shows the structure of one allotropes of carbon.
- Identify the allotrope ( 1 Mk)
- graphite
- graphite
- State one property of the above allotrope and explain how it is related to its structure. (2Mk) .
- it is a lubricant because layers slide over each other//
- a good conductor of both heat and electricity because it has delocalised/mobile electrons
- Identify the allotrope ( 1 Mk)
- What is meant by allotropy? (1 Mk)
- 24cm³ of a solution of 0.1M potassium hydroxide were exactly neutralized by 30cm³ of a solution of sulphuric acid. Find the molarity of the acid. (3 Marks)
- 2KOH(aq) + H2SO4(aq) → K2SO4(aq) + 2H2O ✓½
0.0024 0.0012 - Moles of KOH(aq) =
2.4 x 0.1 = 0.0024
1000 - Moles of H2SO4(aq) =
1/2(0.0024)
= 0.0012 ✓½ - Molarity of H2SO4 =
0.0012 x 1000
30
= 0.04M ✓½ (3mks)
- 2KOH(aq) + H2SO4(aq) → K2SO4(aq) + 2H2O ✓½
-
- Give one use of hygroscopic substances in the laboratory. (1 Mark)
- used as drying agents for wet gases/test presence of water
- used as drying agents for wet gases/test presence of water
- What is meant by the terms: (2 Marks)
- Isotopes
- Isotopes are atoms with same number of protons/atomic numbers but different number of neutrons/mass numbers
- Isotopes are atoms with same number of protons/atomic numbers but different number of neutrons/mass numbers
- Mass number
- Mass number is the total number of number of protons and neutrons in an atom.
- Mass number is the total number of number of protons and neutrons in an atom.
- Isotopes
- The formulae for a chloride of phosphorus is PCl3. What is the formula of its sulphide? (1 Mk)
- P2S3
- P2S3
- Give one use of hygroscopic substances in the laboratory. (1 Mark)
- The diagram below shows the Frasch process used for extraction of sulphur. Use it to answer the questions that follow.
- Identify X. (1mk)
- Hot compressed air.
- Hot compressed air.
- Why is it necessary to use super heated water in this process? (1mk)
- To melt sulphur
- To melt sulphur
- State two physical properties of sulphur that makes it possible for it to be extracted by this method. (1mk)
- Low melting point
- Does not dissolve in water (1mk)
- Identify X. (1mk)
- A certain carbonate XCO3 , reacts with dilute hydrochloric acid according to the equation given below:
XCO3(s) +2HCl (aq) → XCl2(aq) + CO2(g) + H2O(l)
If 4g of the carbonate reacts completely with 40cm3 of 2M hydrochloric acid, calculate the relative atomic mass of X. (C=12.0 ,O=16.0, Cl=35.5). (3 Mks)- No. Of moles of HCl in 40cm3 of 2M HCl
= 40cm³x2M
1000
=0.08moles
Mass of HCl in 0.08moles = 36.5 x 0.08
= 2.92g
4g of the XCO3 reacts with 2.92g 0f HCl.
X + 60g of XCO3 reacts 2 x 36.5g of HCl
2.92(X+60)g=4x2x36.5g
2.92X+175.2 = 292
2.92X=292-175.2
=116.8
X= 116.8/2.92
=40
- No. Of moles of HCl in 40cm3 of 2M HCl
- Concentrated sulphuric acid is slowly added to a mixture of freshly prepared solution of iron (II) sulphate and potassium nitrate as below.
- State the observation made. (1mk)
- A brown ring
- A brown ring
- State the observation made. (1mk)
- The table below gives some properties of three substances I, J and K. Study it and answer the questions that follow.
- Suggest the type of structure in
- I Giant metallic ✓¹ Reject metallic (1mk)
- K Giant ionic ✓¹ Reject ionic (1mk)
- Explain why the molten K is decomposed by electric current but I is not decomposed. (2mks)
- K is an ionic compound while I is a a metallic element with mobile electrons
- Suggest the type of structure in
Download Chemistry Paper 1 Questions and Answers - Form 4 Opener Term 1 Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students