CHEMISTRY
PAPER 2
Instructions
- Attempt all the questions in the spaces provided.
Questions
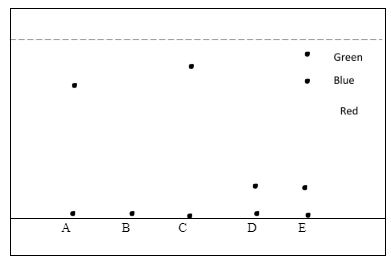
- The diagram below shows chromatograms for five different dyes.
- Name the technique used to separate the dyes. (1 mk)
- What conditions are required to separate the chromatograms present in a dye? (2 mks)
- What is meant by the term solvent front? Indicate its position in the diagram. (1 ½ mks)
- Which chromatogram were present in dye D. (1 mk)
- Which dye is insoluble? (1 mk)
- Which dye is pure? Explain. (1 ½ mks)
- Which chromatogram is most soluble? (1 mk)
- How can one obtain the extract of the blue dye? (2 mks)
- The results showed that dye E contained unwanted colour. Identify the colour. (2 mks)
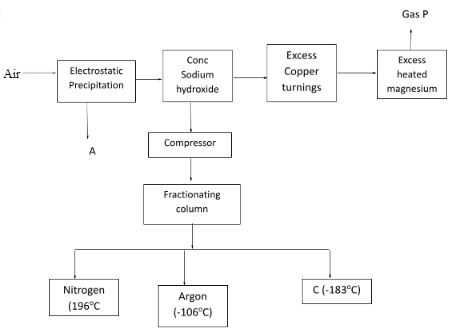
- Air was passed through several reagents as shown in the flow chart below.
- Write an equation for the reaction which takes place in the chamber with.
- Concentrated Sodium hydroxide. (1 mk)
- Excess heated copper turnings. (1 mk)
- Excess heated magnesium (1 mk)
- Name one gas which escapes from the chamber containing magnesium powder. Give a reason for your answer. (2 mks)
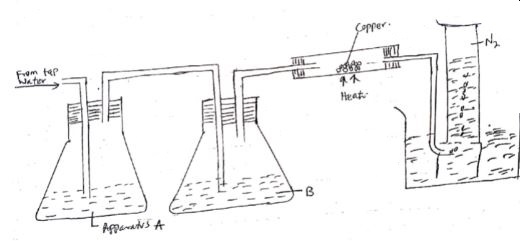
- The diagram below shows an experimental set up for the laboratory preparation of nitrogen gas.
- Name the reagent B and state its role. (2 mks)
- What is the observation made in the combustion tube? (1 mk)
- Nitrogen gas collected using this method is not pure. Explain. (1 mk)
- Give a reason why liquid nitrogen is used for storage of semen for artificial insemination. (1 mk)
- Write an equation for the reaction which takes place in the chamber with.
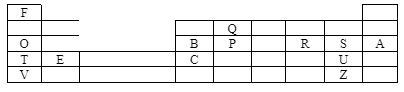
- Use the grid below to answer the questions that follow. The letters do not represent actual symbols of the elements.
-
- Which element form ions with a charge of -2? Explain. (2 mks)
- Identify and explain the elements with great tendency of forming covalent compound. (2 mks)
- How do the reactivity of the following elements compare? Explain.
- T and V (2 mks)
- T and E (2 mks)
- S and Z (2 mks)
- Select the element with the largest atomic radius. Give a reason. (2 mks)
-
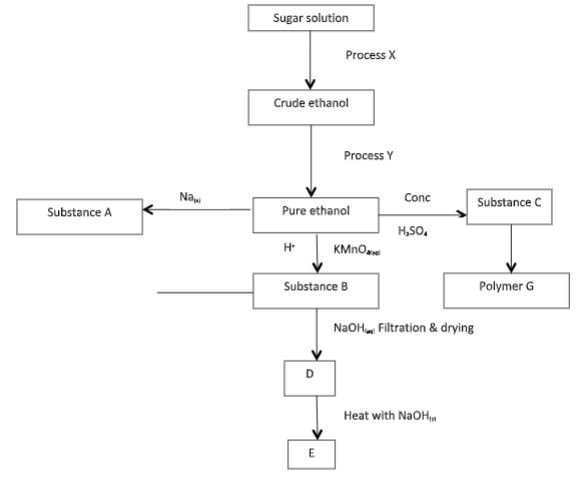
- Study the flowchart below and answer the questions that follow.
- Identify substances A, B, C and E. (2 mks)
- Identify process X and Y. (1 mk)
- Write chemical equations to show how substance E may be obtained from B. (2 mks)
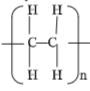
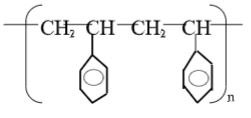
- Draw the structure of; (2 mks)
- Polymer G
- The repeating unit of polymer G
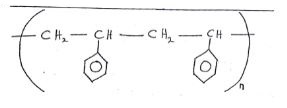
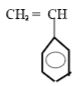
- A polymer is represented by:
- Draw the structure of the monomer. (1 mk)
- A sample of the polymer was found to have relative molecular mass of 3952. Determine the volume of n. (H = 1.0, C = 12.0) (2 mks)
- State one demerits of this kind of polymer as a synthetic fibre. (1 mk)
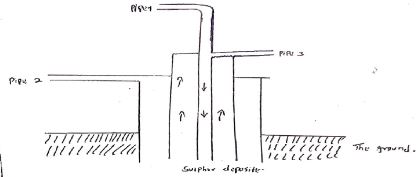
- The diagram below shows the extraction of Sulphur by the Frasch process.
- State what passes through pipe 1 and 3. (2 mks)
- Give the purpose of the solution that passes through pipe 2. (2 mks)
- The following equation is the reaction of Sulphur (iv) oxide and oxygen gas. Use it to answer the questions below.
2SO2(g) + O2(g) ⇌ 2SO3(g)- Name the catalyst used for this reaction. (1 mk)
- Explain briefly how Sulphur (vi) oxide is converted to Sulphuric (VI) acid in the contact process. (2 mks)
-
- State two effects of Sulphur (iv) oxide on the environment. (2 mks)
- When ammonia is passed through concentrated Sulphuric acid, ammonium sulphate fertilizer is produced.
- Write an equation for the reaction. (1 mk)
- Calculate the mass in kg of Sulphuric acid required to produce 250kg of the ammonium sulphate fertilizer (S = 32.0 O = 16.0, N = 14.0, H = 1.0) (3 mks)
- A sample of hydrated Iron (II) sulphate weighing 6.8g was dissolved in water and the solution made up to 250cm3. 25cm3 of the solution was titrated against a solution of 0.02 molar acidified potassium manganite (VII). The titre volumes obtained were 22.8cm3, 22.4cm3 and 22.5cm3 for the first, second and third titrations.
-
- Complete the following table. (3 mks)
Final burette reading (cm3) 1 2 3 Initial buretter reading (cm3) 0.00 0.00 22.4 Total titre volume (cm3) 4 5 6 - Determine the average titre volumes (1 mk)
- Complete the following table. (3 mks)
- Determine the number of moles of manganese (VII) ions used. (2 mks)
- Calculate:
- The concentration of the iron (II) sulphate in mol/dm3 (molar mass = 278) (1 mk)
- Moles of iron (II) ions in 25cm3 of solution. (2 mks)
- An indicator is not required for this titration. How can the end point be determined? (2 mks)
-
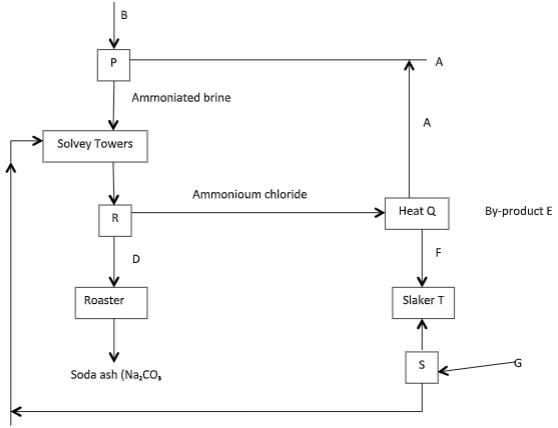
- The table below shows industrial manufacture of sodium carbonate. Study it and answer this questions that follow.
- Name the raw materials A and B. (2 mks)
- Write equations for the reactions taking place at Q and S. (2 mks)
- Name recycled substances and include equations for the reactions. (2 mks)
- Name processes S and R. (2 mks)
- Name by-product E. (1 mk)
- Name substance D. (1 mk)
Marking Scheme
-
- Chromatography
-
- The chromatogram must have different solubility rate
- The dye must be soluble in the given solvent
- It is the furthest distance reached by the eluting solvent on the absorbent material(or filter paper)
- Red
- dye B
- Dye A and C - They have only one chromatogram
- E- moves furthest
- Cut the part of the filter paper with chromatogram blue in colour. Dissolve the chromatogram usinf a suitable solvent. Allow the solution to ecaporate to dryness to obtain solute that is blue in colour
- Green - it does not mix with any other colour of the chromatograms.
-
-
- CO2(g) + NaOH(aq) → NaHCO3(aq)
- 2Cu(s) + O2(g) → 2CuO(s)
- 3Mg(s) + N2(g) → Mg3N2(g)
- Neon, argon , helium
They are stable and thus unreactive
-
- Concentrated KOH or concentrated NaOH
It is used to absorb Carbon (IV) oxide - Red brown copper changes colour to black copper (II) oxide
- It contains traces of noble gases
- Liquid nitrogen has a low boiling point. It provides low temperatures required for semen storage.
- Concentrated KOH or concentrated NaOH
-
-
-
- R- It gains two electrons to attain noble gas structure
- Q - It has the ability to bond with itself many times , a process known as catenation.
-
- T and V
- V is more reactive than T: T has bigger radius hence less nuclear pull. The nucleus of V is screened, thus less energy required to lose electrons
- T and E
- T is more reactive than E. T has fewer protons than E leading to less nucleat pull and bigger radius, thus T requires less energy to lose electrons
- S and Z
- S is more reactive than Z, S has a smaller radius leading to a higher effective nuclear charge compared to Z: the nucleus of S is less screened.
- V- It has the largest number of energy levels and the fewer number of protons within period.
- T and V
-
-
-
- A- Sodium ethoxide
- B - Ethanoic acid
- C- Ethene
- D - Sodium ethanoate
-
- X- Fermentation
- Y- Fractional distilation
- CH3COOH + NaOH(aq) → CH3COONa + H2O
CH3COONa + NaOH → CH4 + Na2CO3 -
-
-
-
- Molecular mass of monomer: (d x 1) + (8 x 12)=104
Value of n = 395/104 =38 - It is non- biodegradable therefore it possesses a problem of disposal.
-
-
-
- 1 - Hot compressed air under pressure
3 - Molten sulphur - To heat and melt sulphur from solid into molten sulphur
-
- Vanadium (V) oxide
- Sulphur (vi) oxide is cooled and dissolved in concentrated sulphuric acid in the absorption tower to oleum. Oleum is diluted with water to concentrated sulphuric (vi) acid.
H2S2O7 + H2O → 2H2SO4(l)
-
- Sulphur (IV) oxide is oxidised in the atmosphere to Sulphur (vi) oxide, a secondary pollutant, which dissolves in water to sulphuric acid causing acid rain. Acid rain causes corrosion odf building metals. -SO2 when inhaled causes lung cancer in humans.
-
- -2NH3(aq) + H2SO4(l) → (NH4)SO4 (a slo accept this)
-2NH4OH + H2SO4(l) → (NH4)SO4 + 2H2O - 1 mole H2SO4 = 1 mole (NH4)2SO4
98g = (14 x4)2 + 96g
= (36 + 96g)= 132g
98kg = 132kg
98 x 250kg = 185.606kg
132
- -2NH3(aq) + H2SO4(l) → (NH4)SO4 (a slo accept this)
- 1 - Hot compressed air under pressure
-
-
- 1. - 22.8
2- 22.4
3 - 44.9
4 - 22.8
5 - 22.4
6 - 22.5 - (22.4 + 22.5) = 22.45cm3
2
- 1. - 22.8
- Moles = M x Vol/cm3
1000
(0.02 x 22.45cm3) = 0.000449moles
1000 - Cal culate:
- Moles = Mass/RAM = 6.28/278 = 0.02259 moles
- Moles=
25 x 0.09036
1000
0.002259 moles
- Moles = Mass/RAM = 6.28/278 = 0.02259 moles
- At the end point the colour changes to pink when all the Iron(II) ions have been converted to Iron (III) ions and manganite (VII) ions have been converted to colourless Mn2+ ions.
-
-
- A- ammonia gas
B - Brine (NACl) - Q- 2NH4Cl(aq) + Ca(OH)2(aq) → CaCl2(aq) + 2NH3 + 2H2O(l)
S- CaCO3(s) → CaO(s) + CO2(g) -
- 2NaHCO3(s) → 2Na2CO3(aq) + H2O(l) + CO2(g)
- 2NH4Cl(aq) + Ca(OH)2(aq) → CaCl2(aq) + 2NH3(g) + 2H2O(l)
- S- Thermal decomposition
R - Filtration - Calcium chloride or CaCl2
- Sodium hydrogen carbonate or (NaHCO3)
- A- ammonia gas
Download Chemistry Paper 2 Questions and Answers - Form 4 Opener Term 1 Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students